 |
| Tauopathies describe a wide range of neurodegenerative diseases associated with tau protein aggregation, with Alzheimer's disease perhaps being the most well-known of them. Prions are proteins that can fold improperly and cause other proteins to conform, leading to aggregation as well. Several scientists are now looking into whether neurodegenerative diseases might be induced by prion-like proteins. Sanders et al. investigated the ability of tau protein to exhibit prion-like functions. BioLegend provides an excellent selection of tau antibodies and recombinant proteins to support these ongoing neurological studies. |
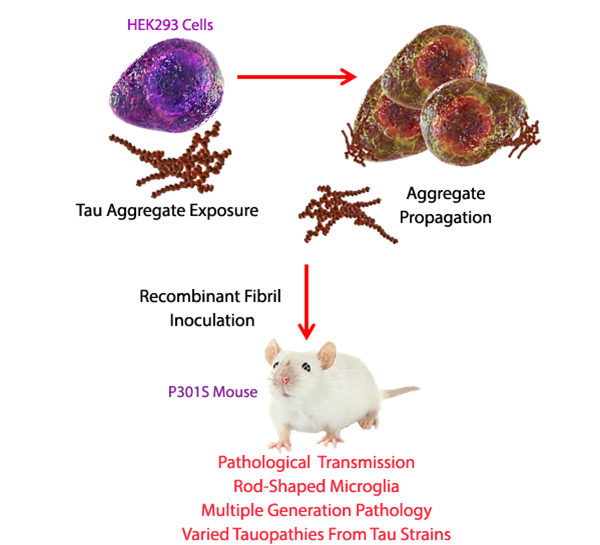 |
| Adapted from: Sanders, D.W. et al. 2014. Neuron. 82:1271. Pubmed |
| Sanders et al. introduced recombinant tau fibrils to the HEK293 cell line, making them indefinitely create aggregated forms of tau. Lysates of these cell lines were then injected into P301S mice, which express a mutant form of human microtubule-associated protein tau that aggregates with age. These mice developed pathological phenotypes and tauopathies. Brain homogenates of these mice could also be passaged and be used to inoculate successive generations of mice. Sanders et al. thus suggest that tau protein should be considered a prion due to these characteristics. |
Related Products |
| Tau Pan-Isoform Antibodies |
| Isoform Specific Antibodies |
| Description |
|---|
| Tau 0N, 3H6.H7 |
| Tau 1N, 4H5.B9 |
| Tau 4R, 5F9 |
| Tau 2N, 71C11 |
| Recombinant Proteins |
| Description |
|---|
| Recombinant Human Tau-352 (0N3R) |
| Recombinant Human Tau-381 (1N3R) |
| Recombinant Human Tau-383 (0N4R) |
| Recombinant Human Tau-410 (2N3R) |
| Recombinant Human Tau-412 (1N4R) |
| Recombinant Human Tau-441 (2N4R) |
*Any references to promotions on this page may not be valid at this time. View our promotions page for the most up-to-date promotions.
 Login / Register
Login / Register 






Follow Us