Phosphorylation and de-phosphorylation play critical roles as a mode of signal transfer in biological processes. This is defined as the transfer of phosphoryl groups [(PO3)2-] from one molecule to another, and serves as a transfer of energy that results in the activation, or deactivation of downstream proteins.
At BioLegend, we offer a wide array of phospho protein-related products for use in flow cytometry, western blotting, and microscopy applications. Whenever possible, the phospho protein-specific antibodies we offer are optimized to work for multiple applications, allowing for clone and product consistency across different experimental systems.
Protein Phosphorylation
PhosphoPair Antibody Sets
PhosphoPair Antibody Sets bundle a pair of antibodies together for cost-savings and convenience. One antibody is specific for a phosphorylated form of a protein, while the other detects total or unphosphorylated levels of the protein. PhosphoPair Antibody Sets are quality tested in western blotting to ensure high quality results in each experiment.
View all PhosphoPair Antibody Sets...
Phosphorylation Targets
The table below lists a number of well known phosphorylated proteins. Learn more about each protein and view the important phosphorylation sites. Also find upstream kinases and phosphatases that interact with the protein.
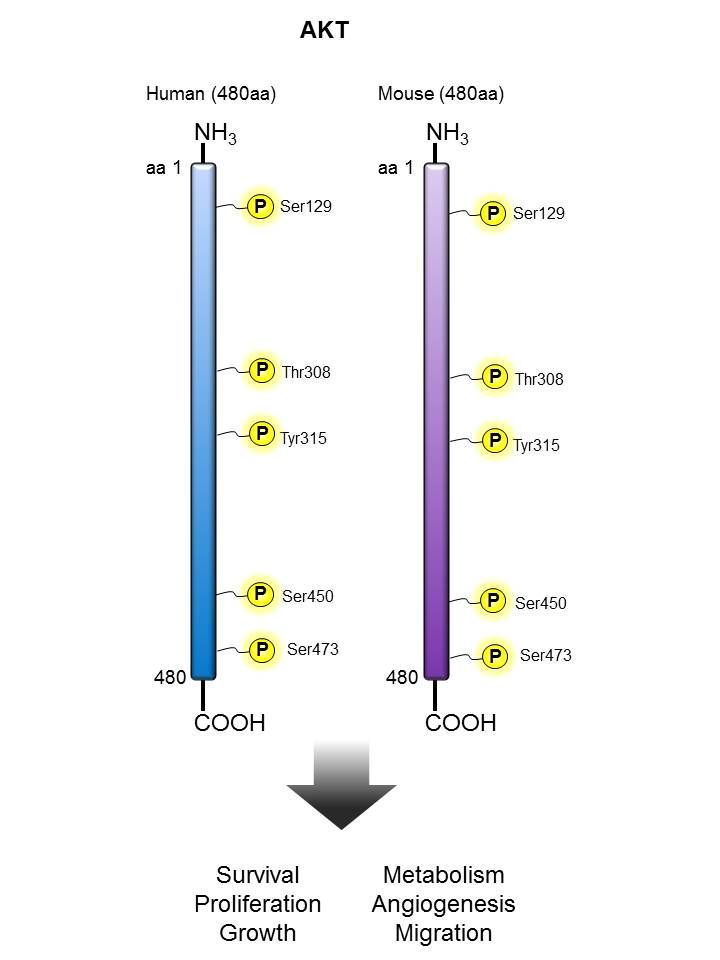
AKT |
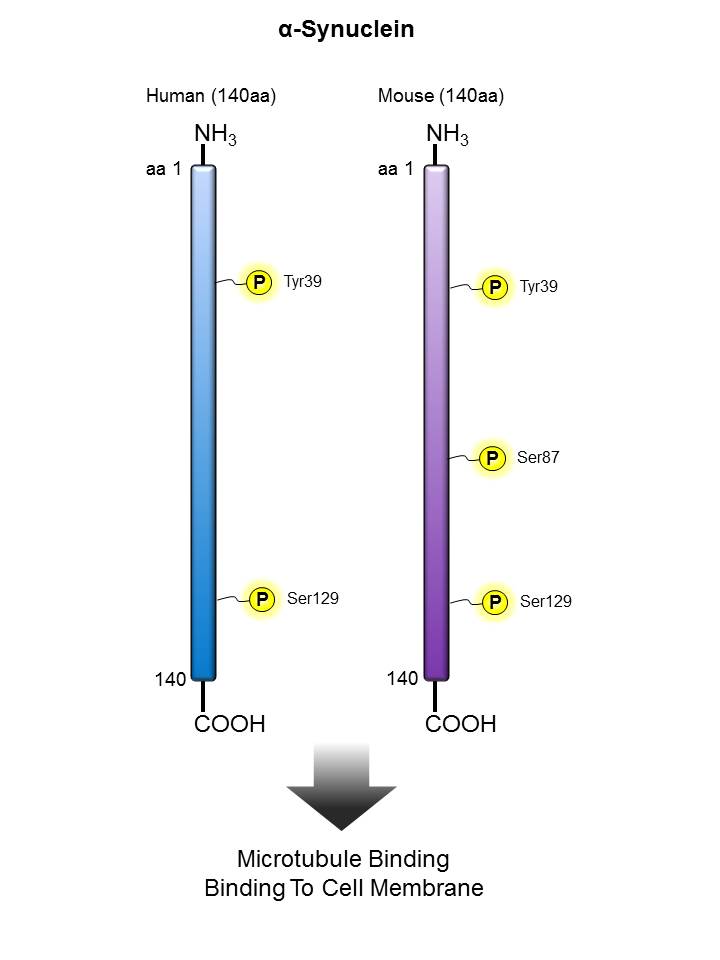
α-Synuclein |
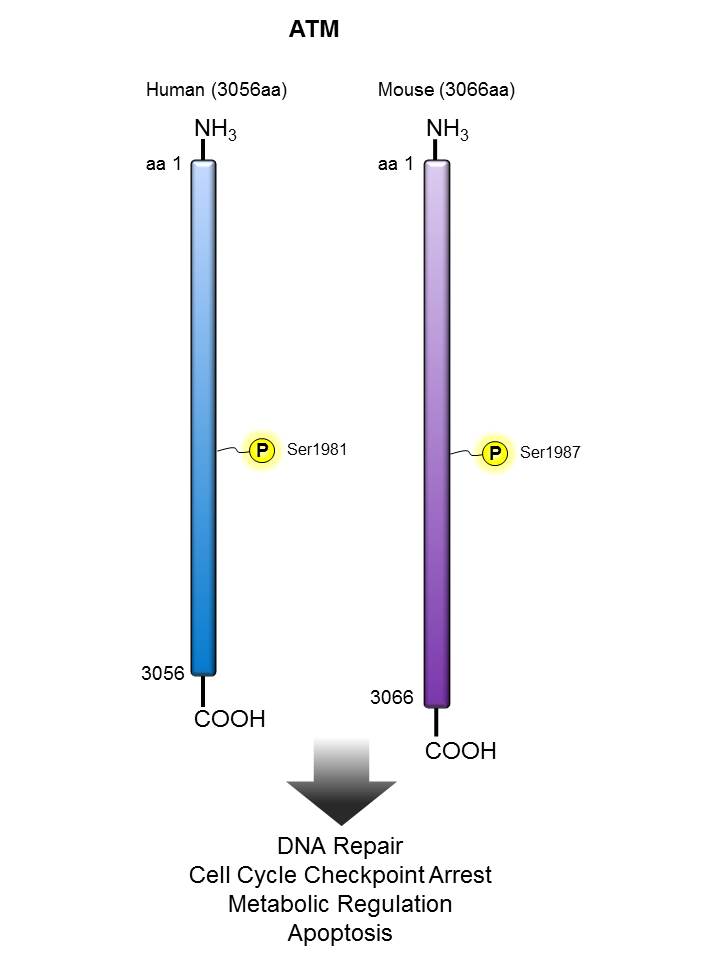
ATM |
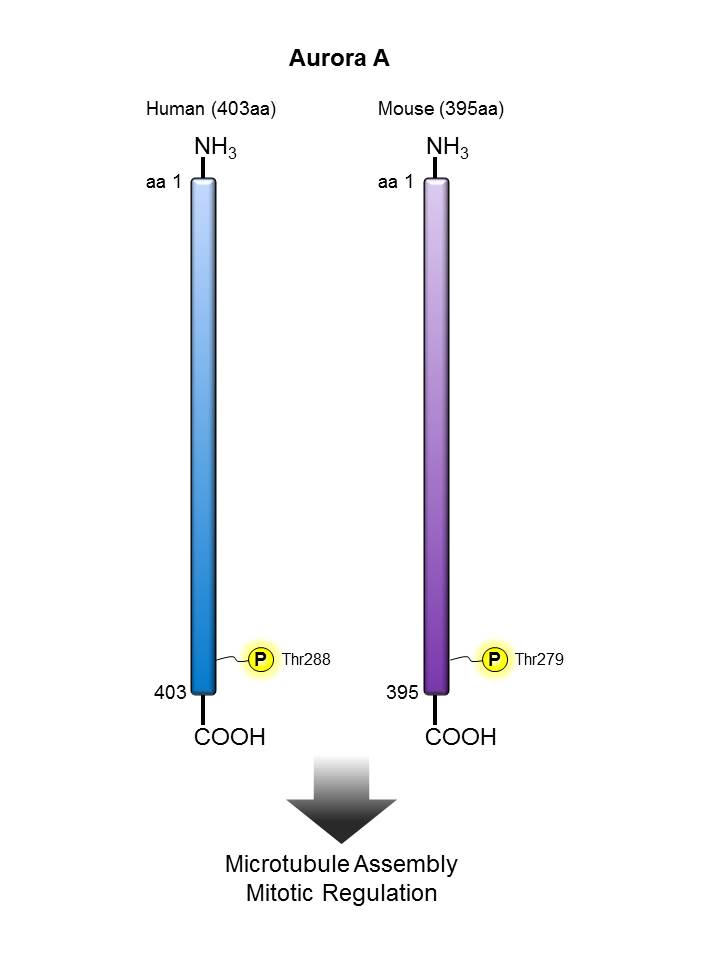
Aurora A |
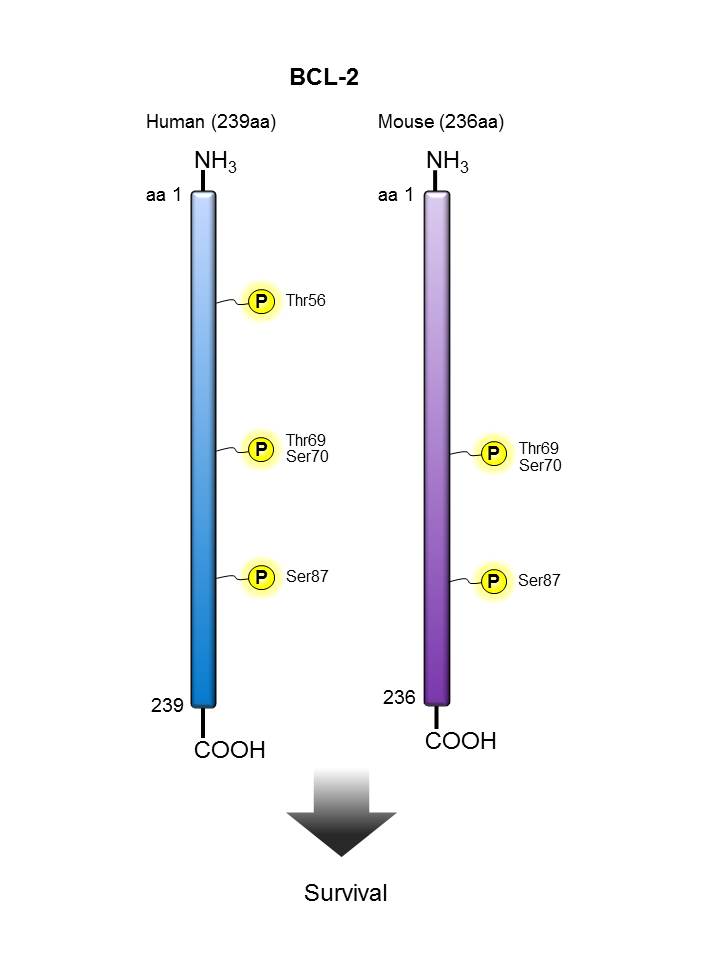
BCL-2 |
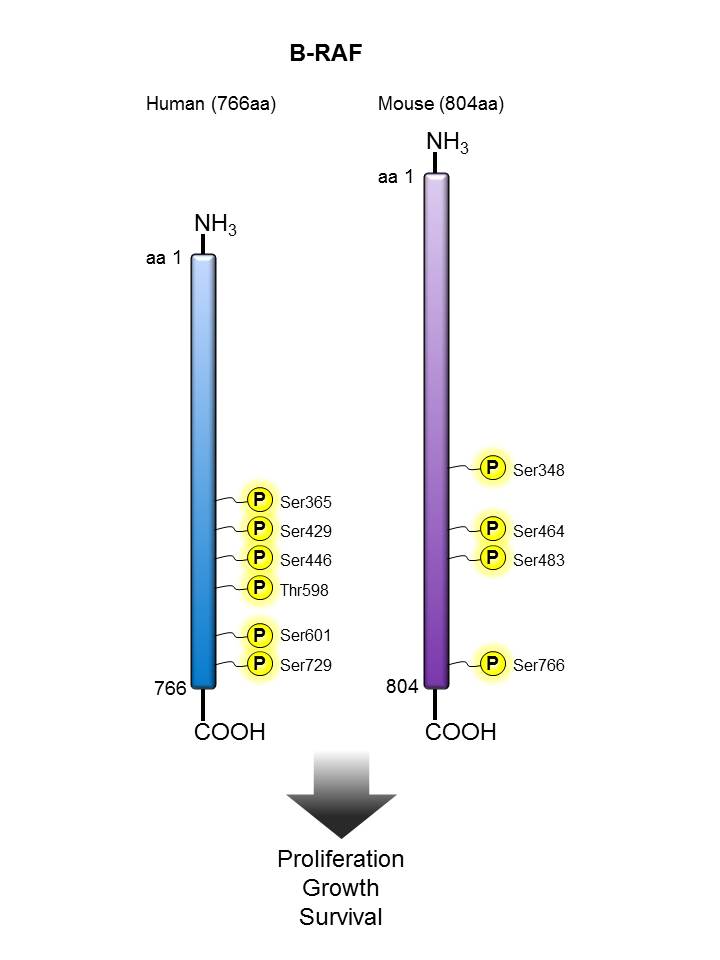
B-Raf |
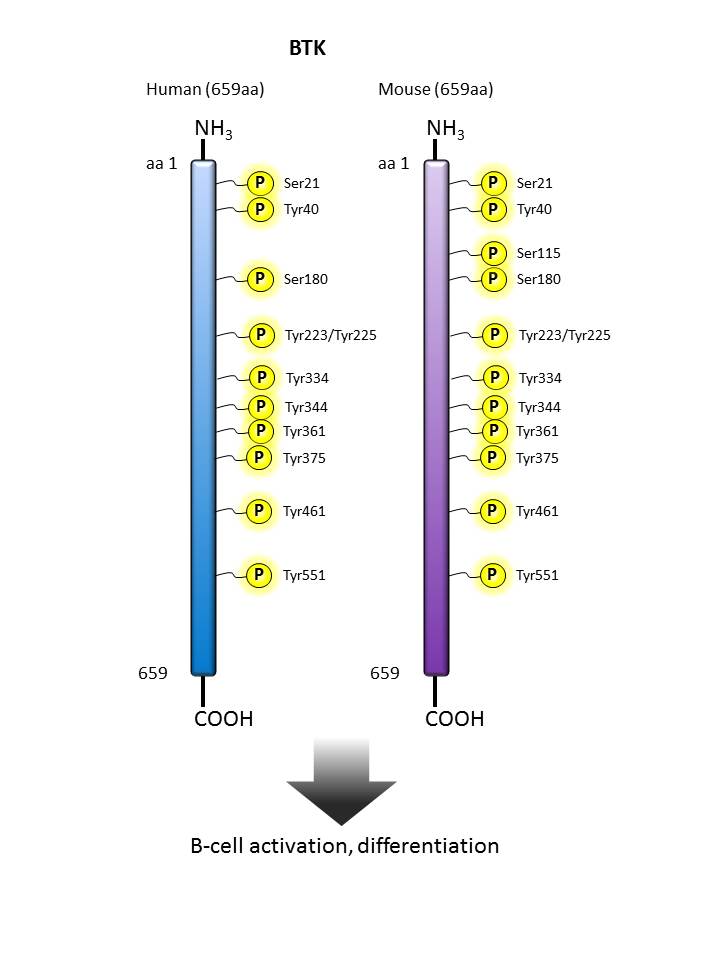
BTK |
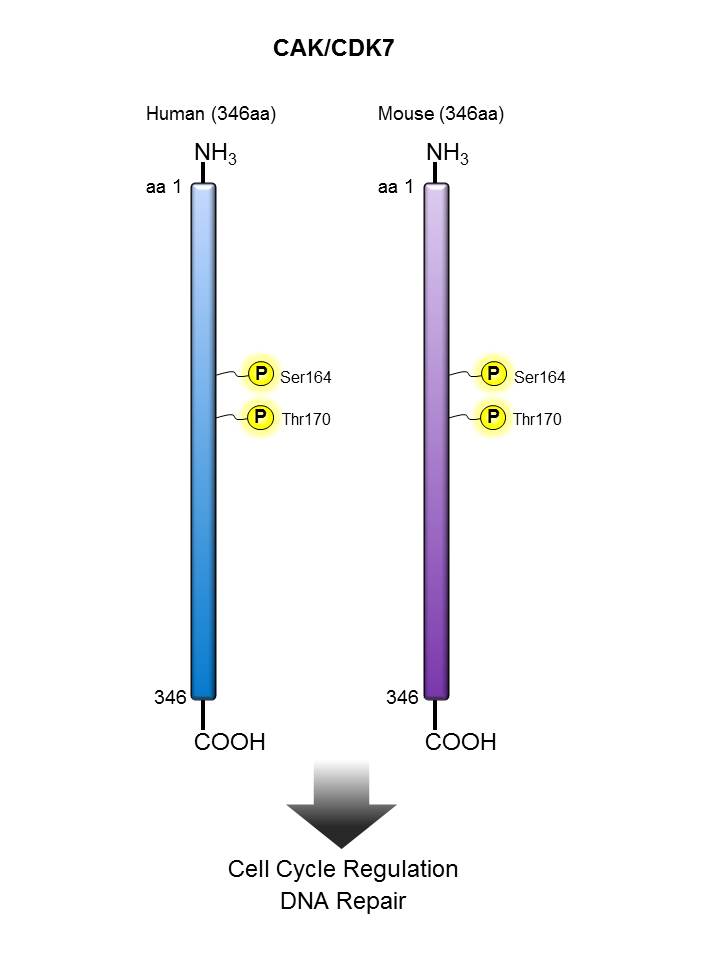
CAK/CDK7 |
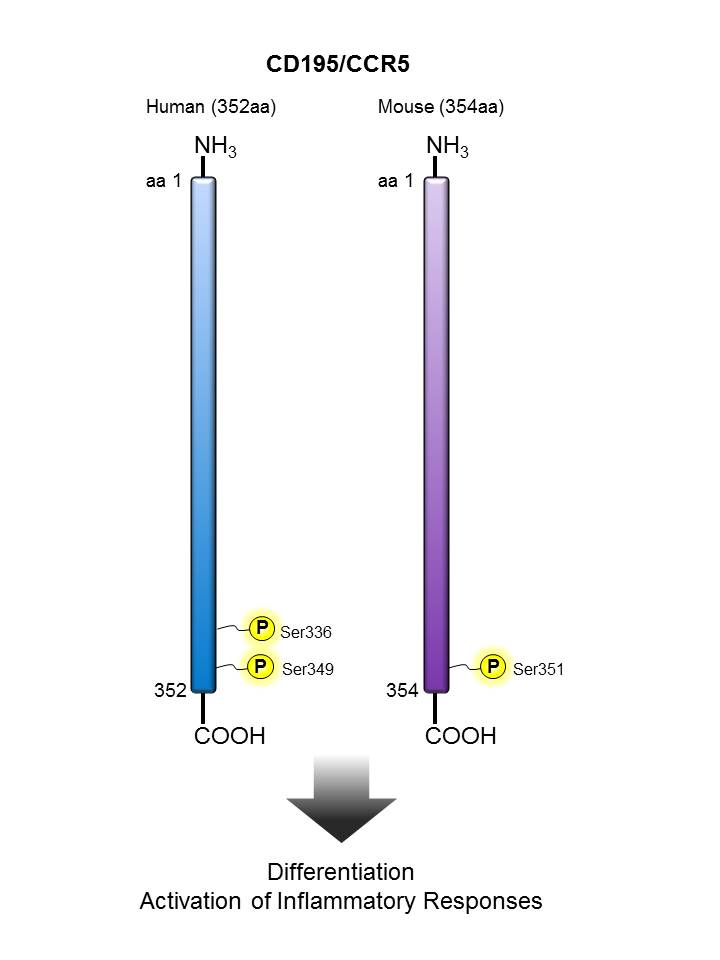
CD195 (CCR5) |
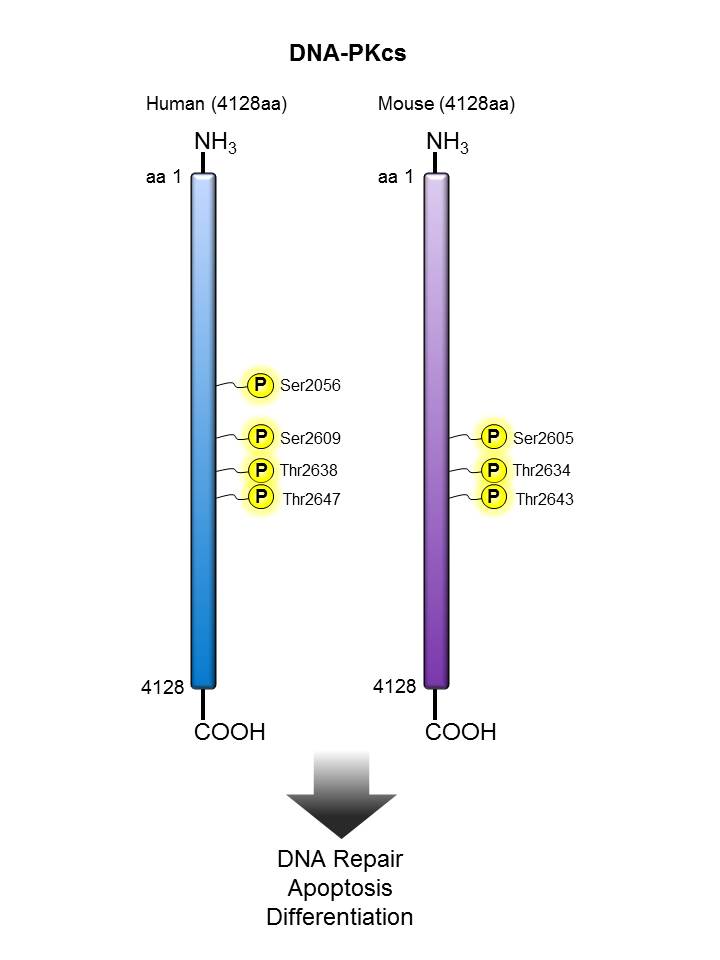
DNA-PKcs |
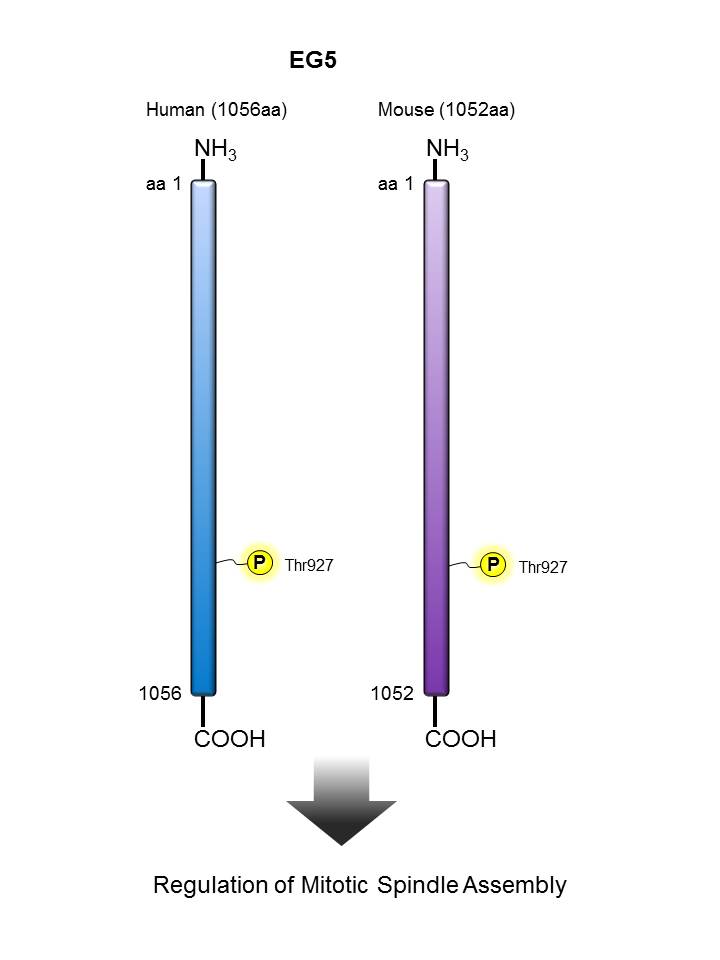
EG5 |
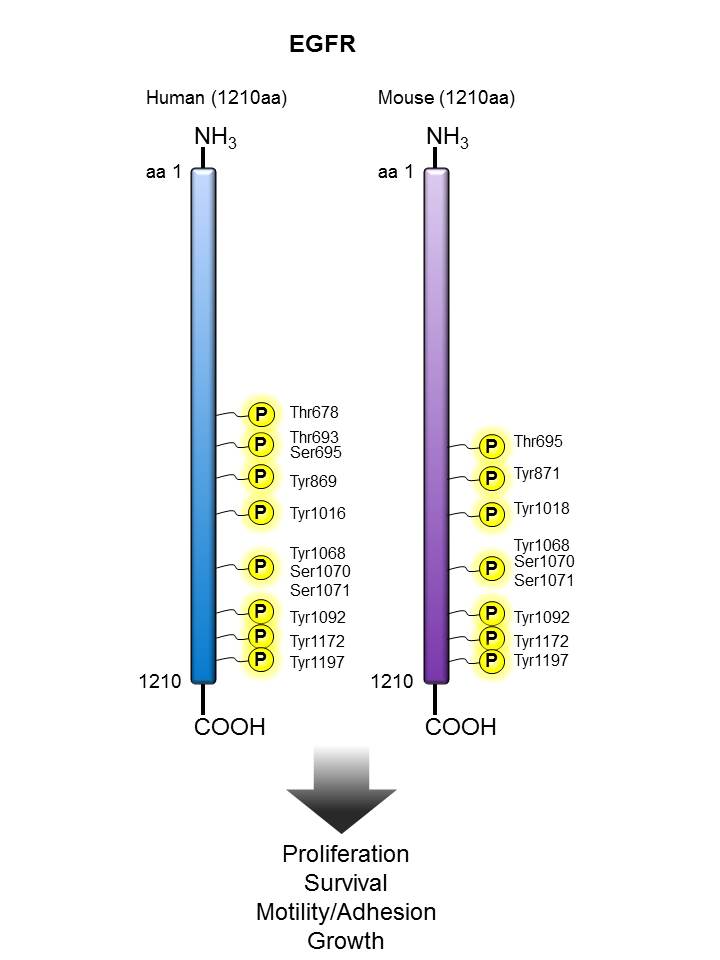
EGFR |
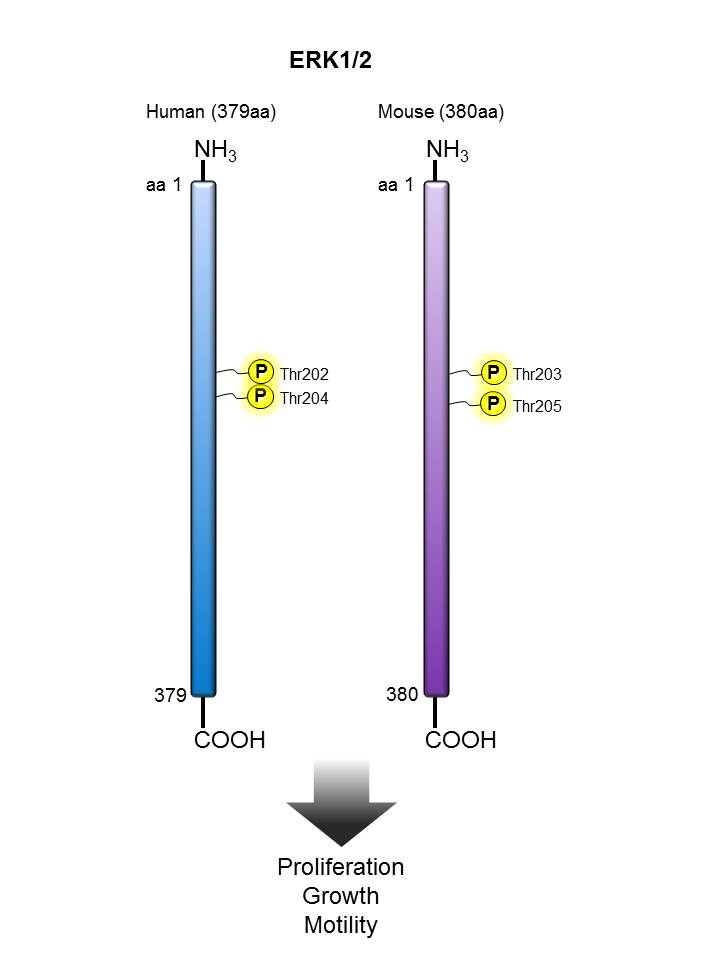
ERK1/2 |
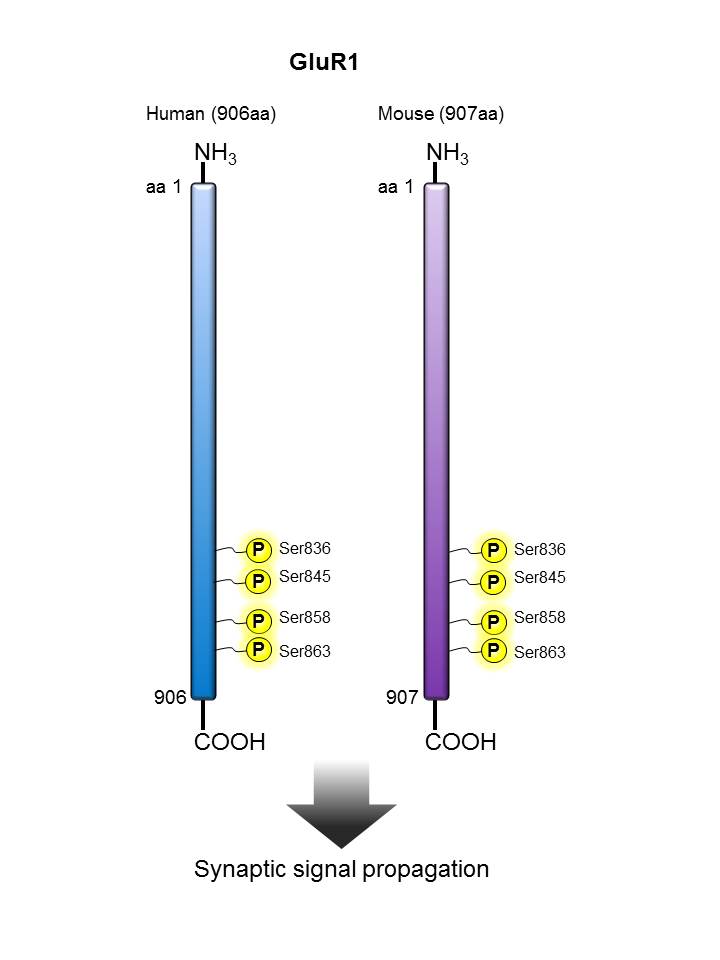
GluR1 |
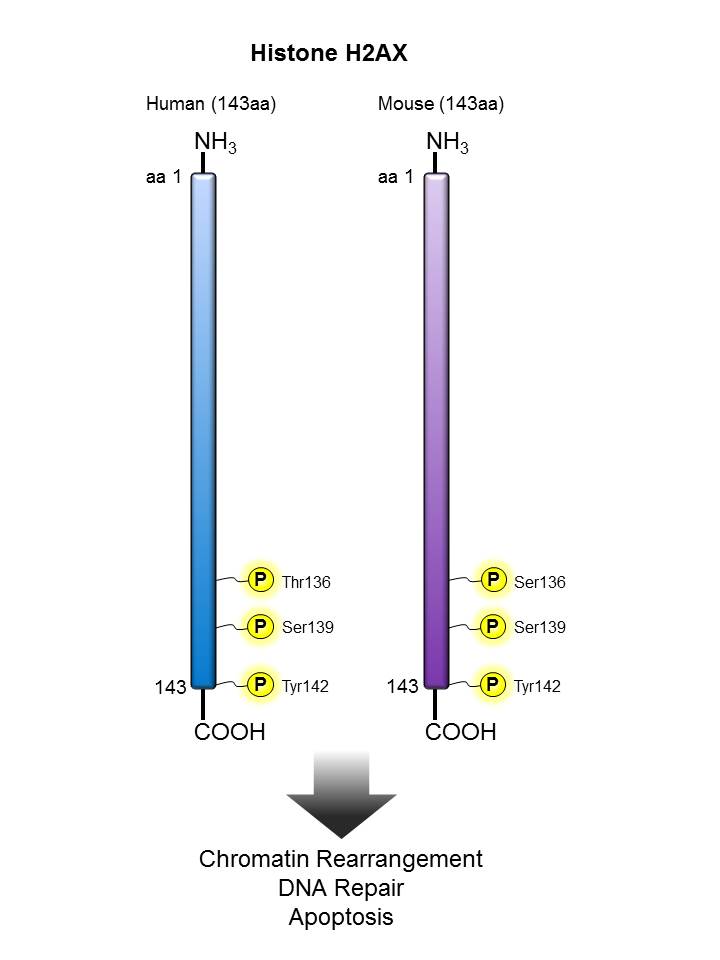
Histone H2AX |
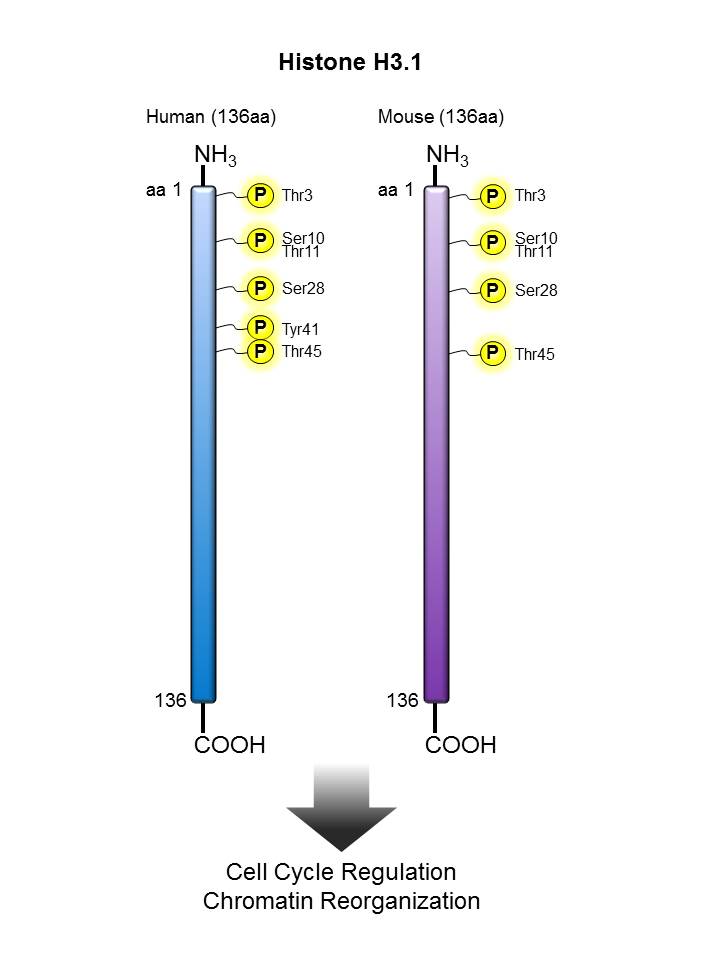
Histone H3.1 |
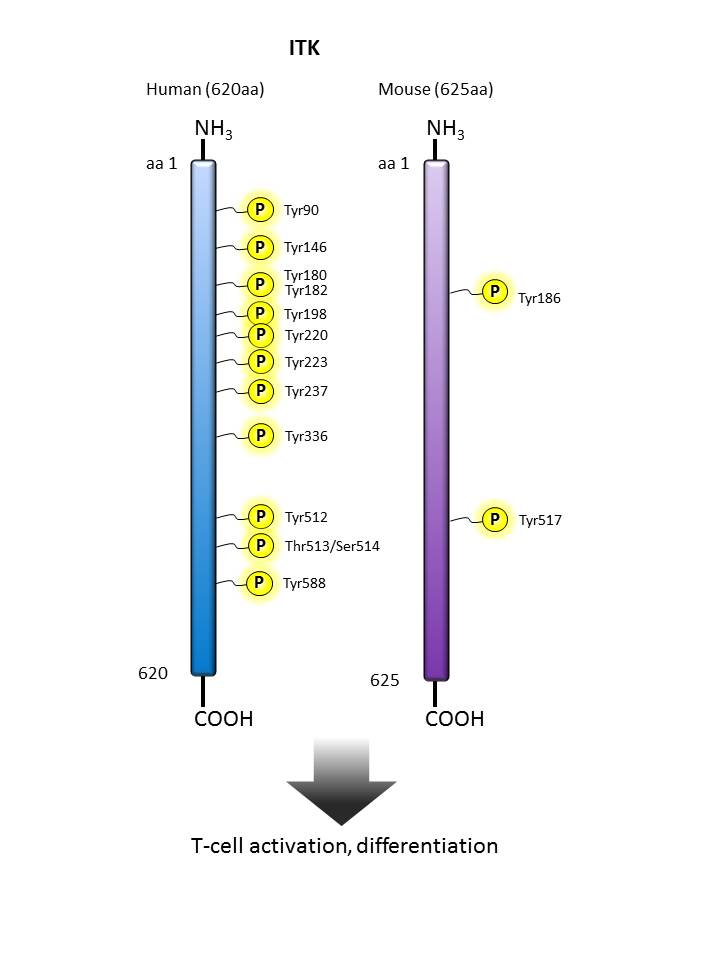
ITK |
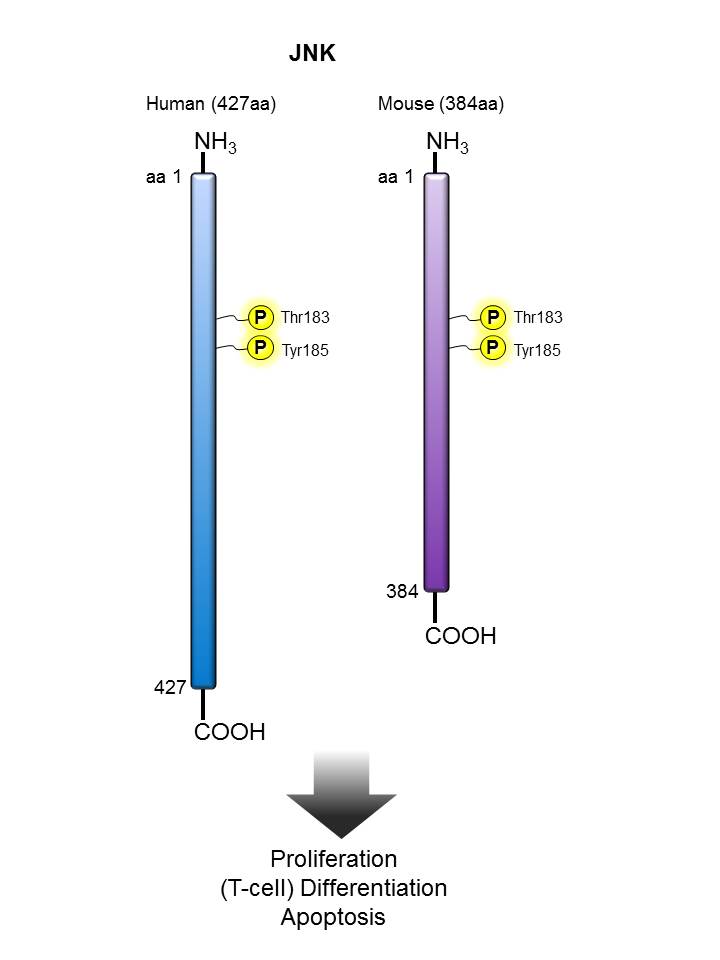
JNK |
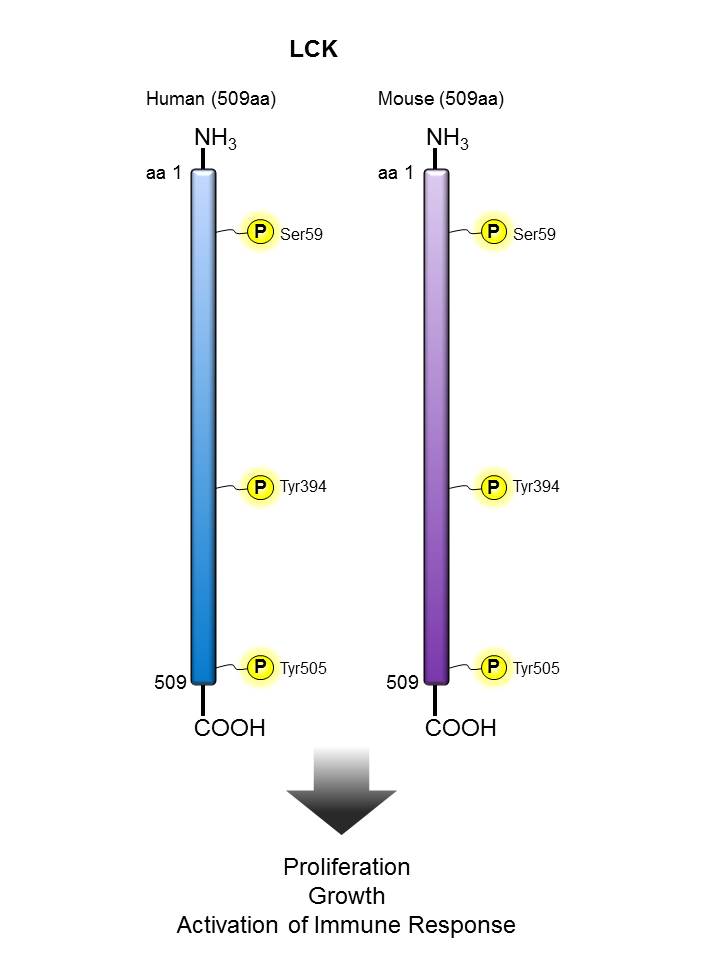
LCK |
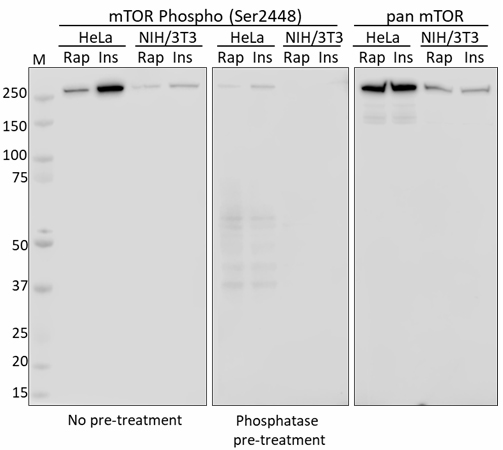
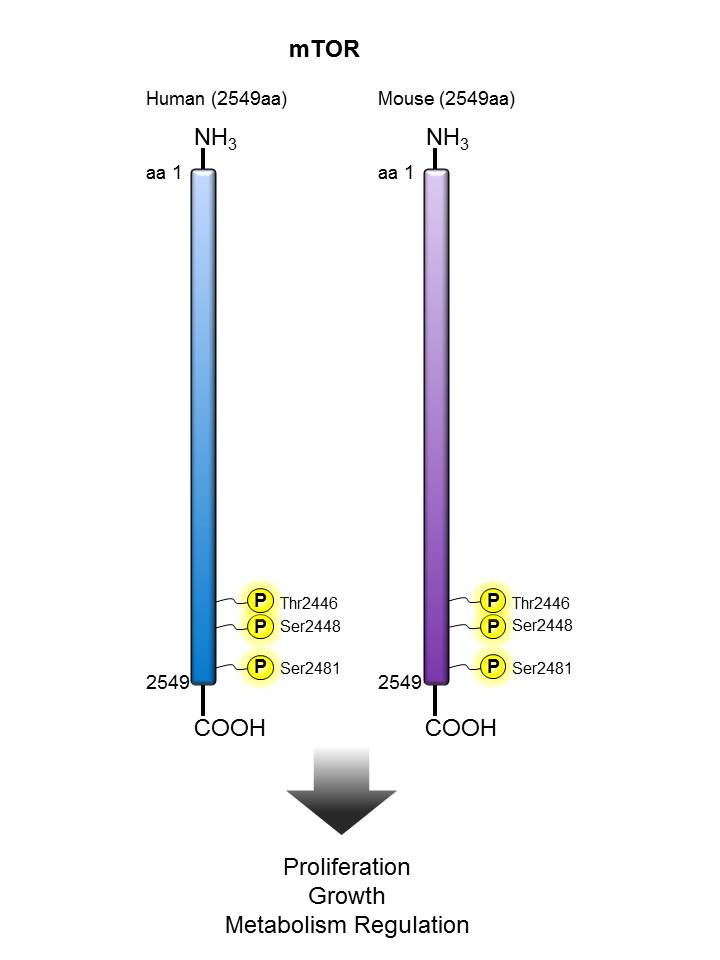
mTOR |
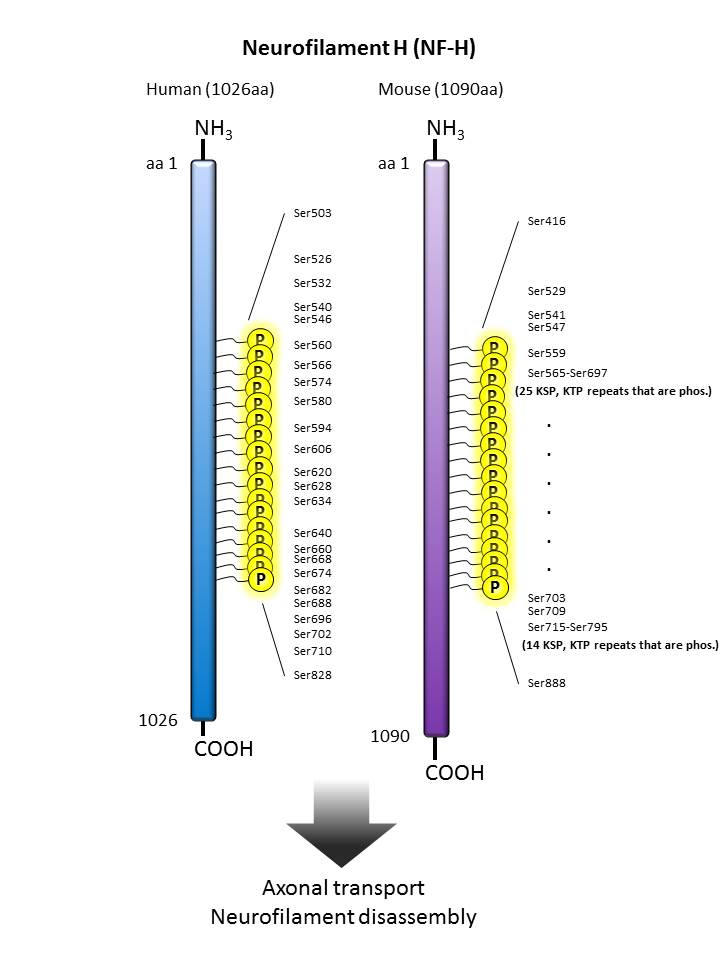
| Neurofilament H  |
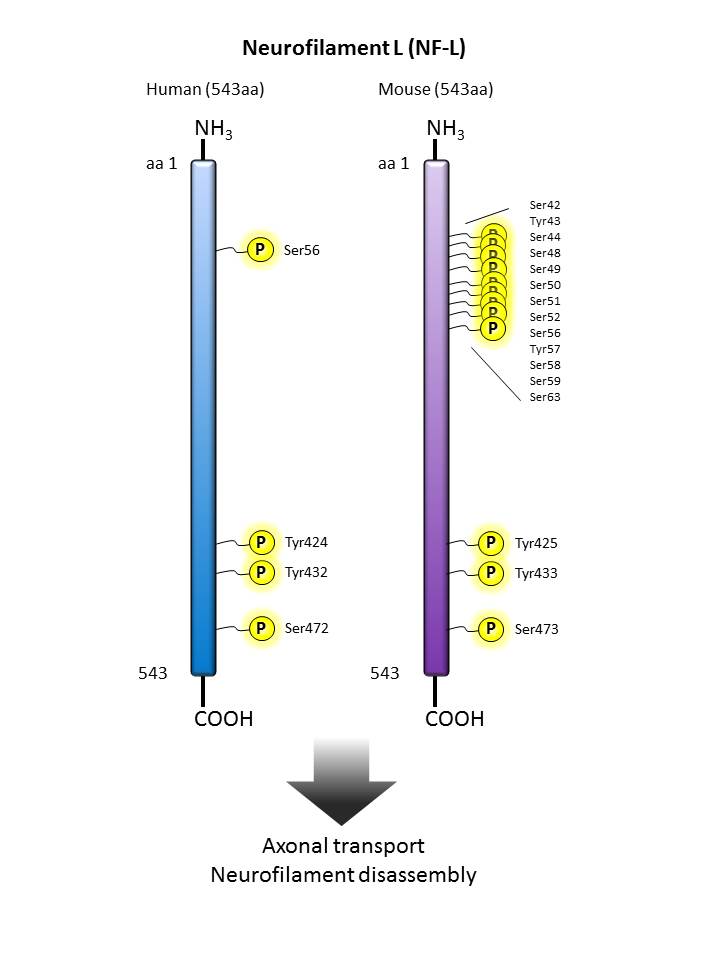
Neurofilament L  |
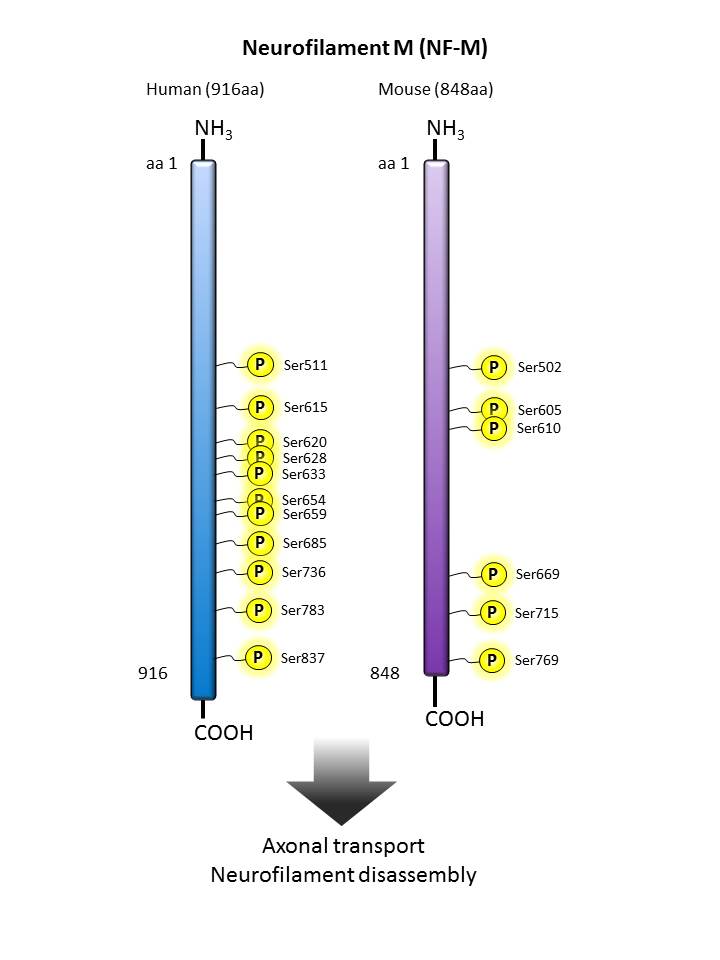
Neurofilament M  |
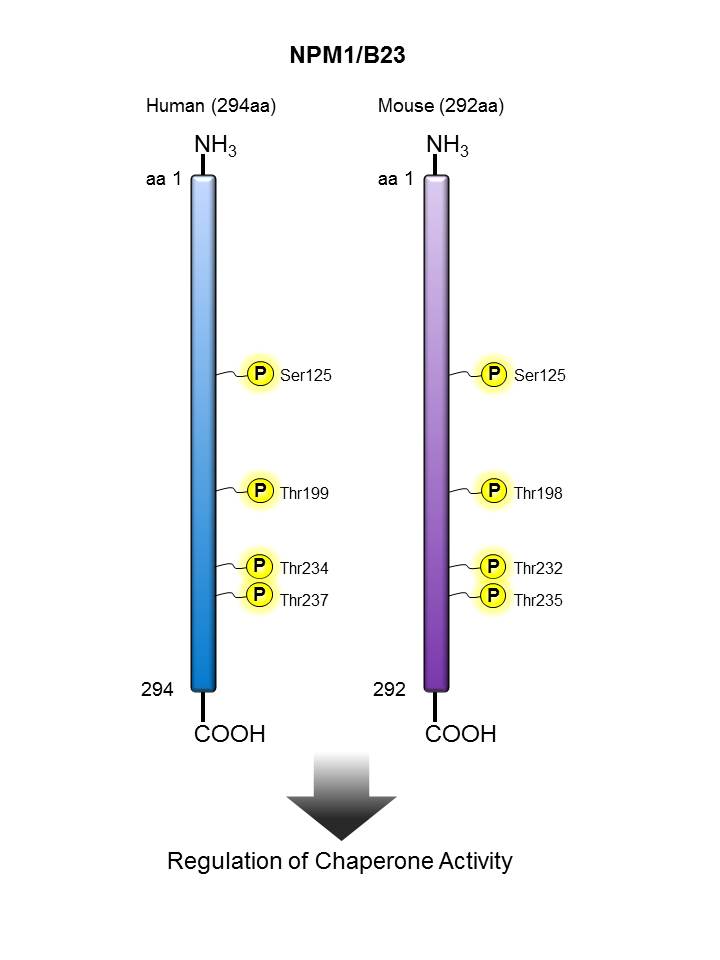
NPM/B23 |
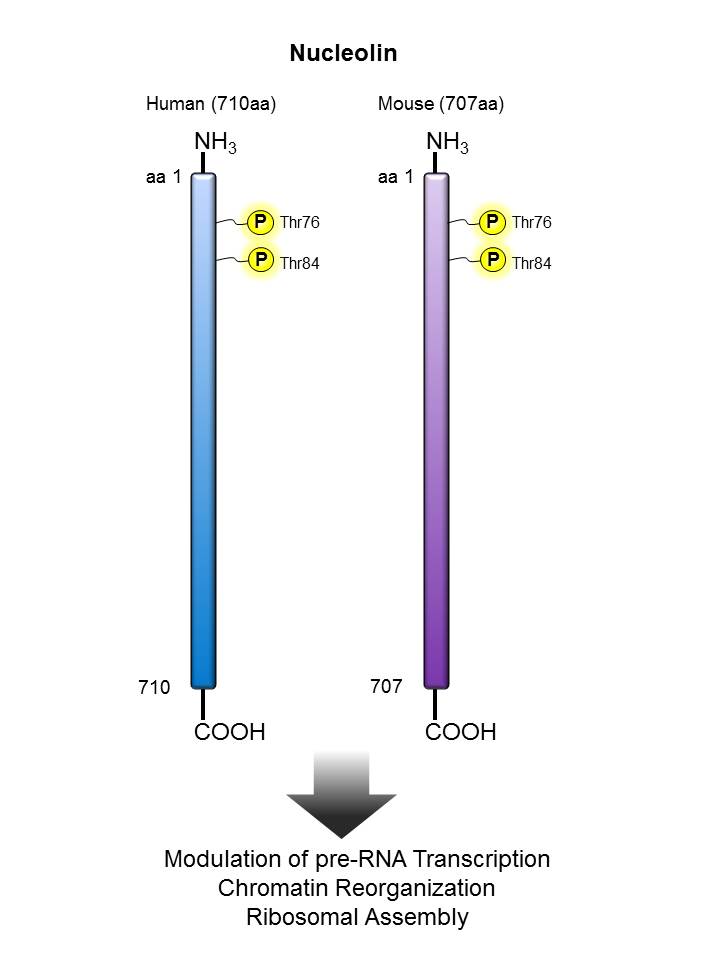
Nucleolin |
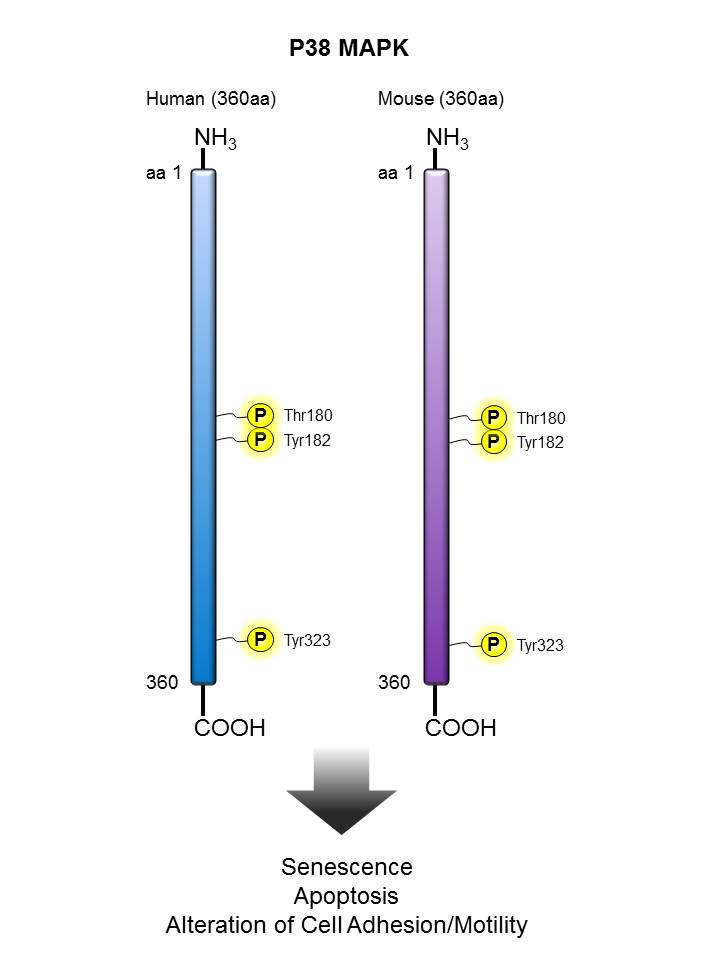
p38 MAPK |
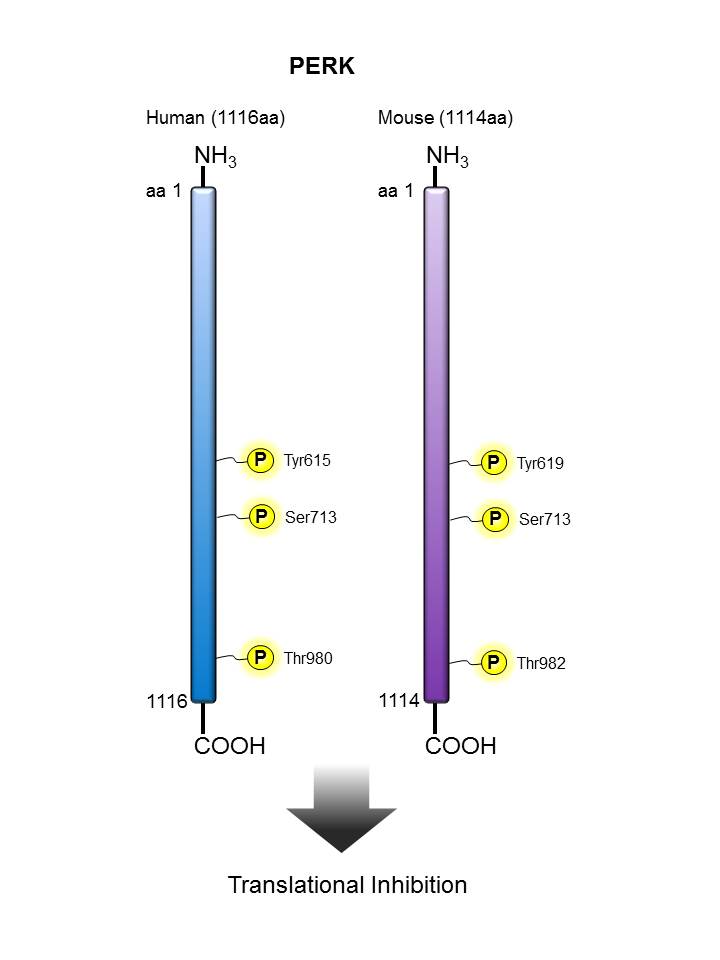
PERK |
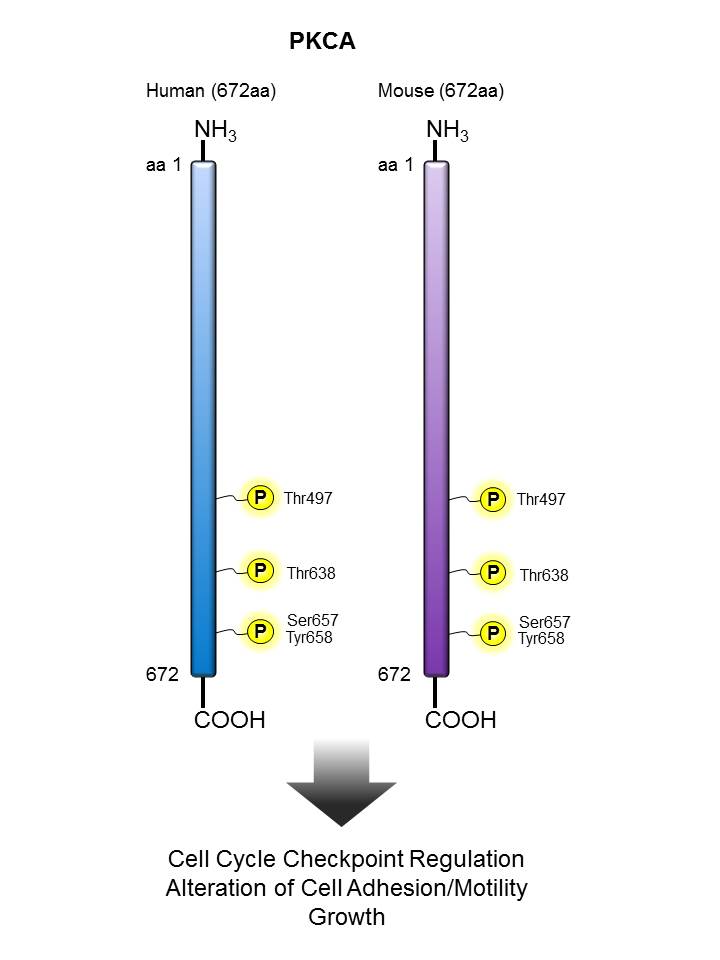
PKCα |
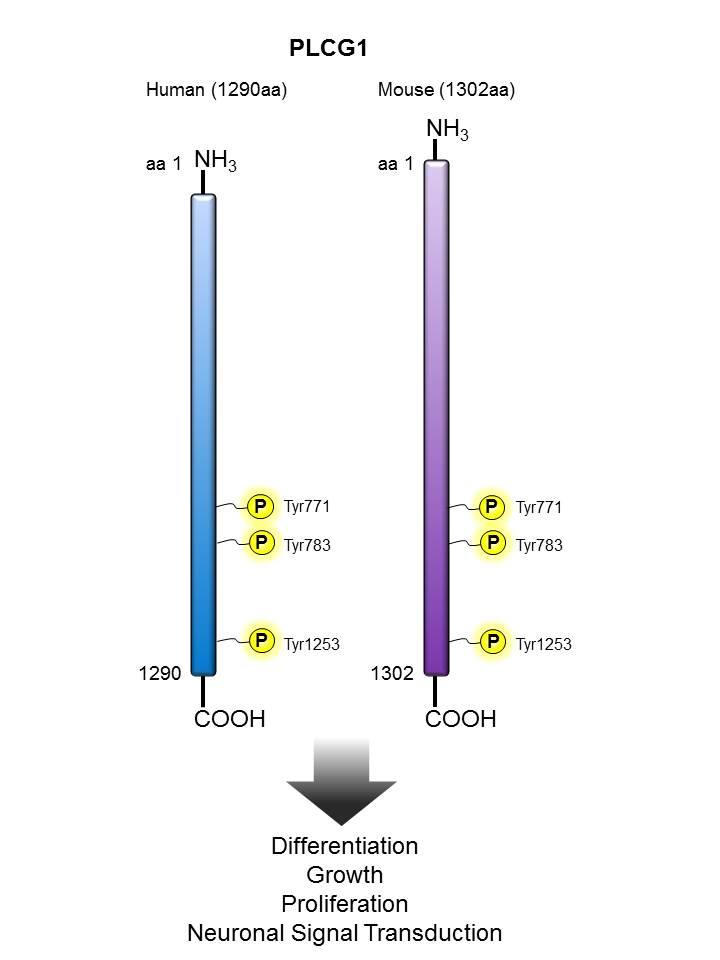
PLCγ |
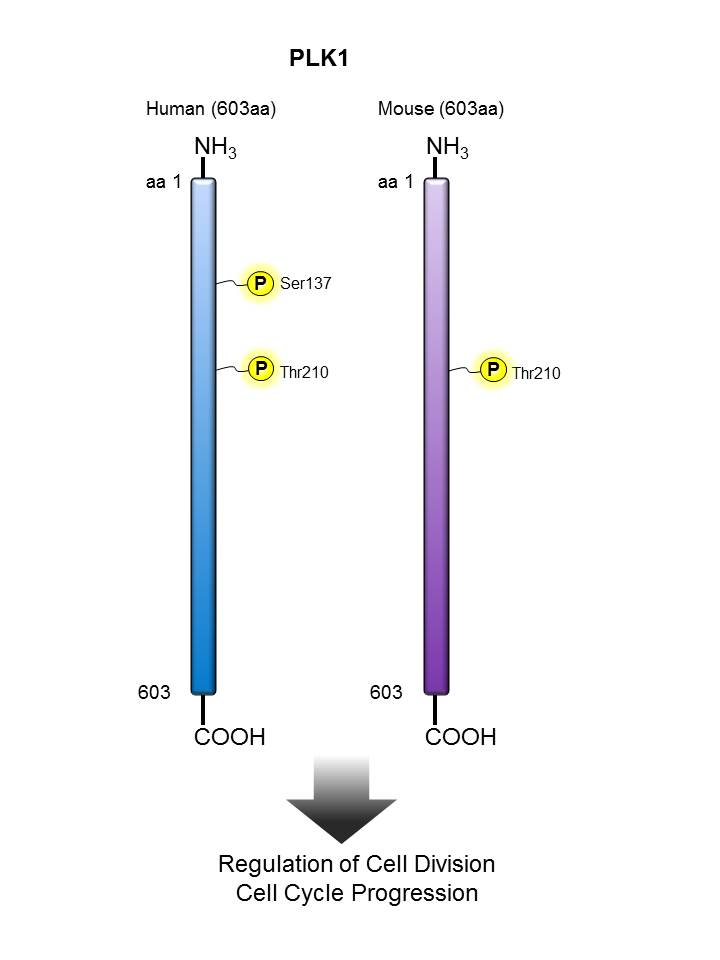
PLK1 |
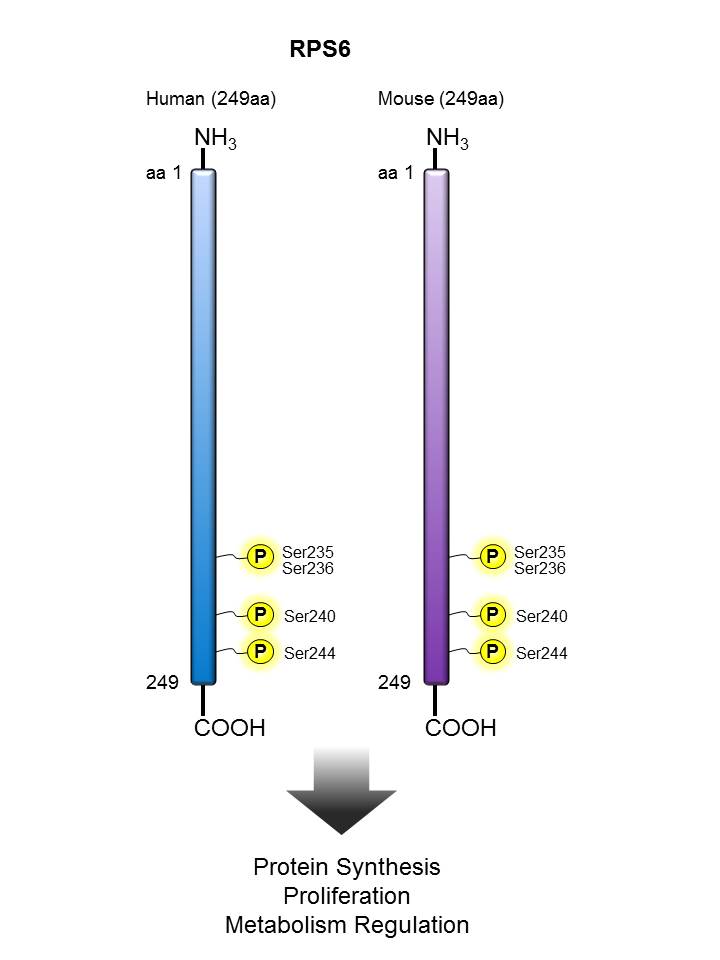
RPS6 |
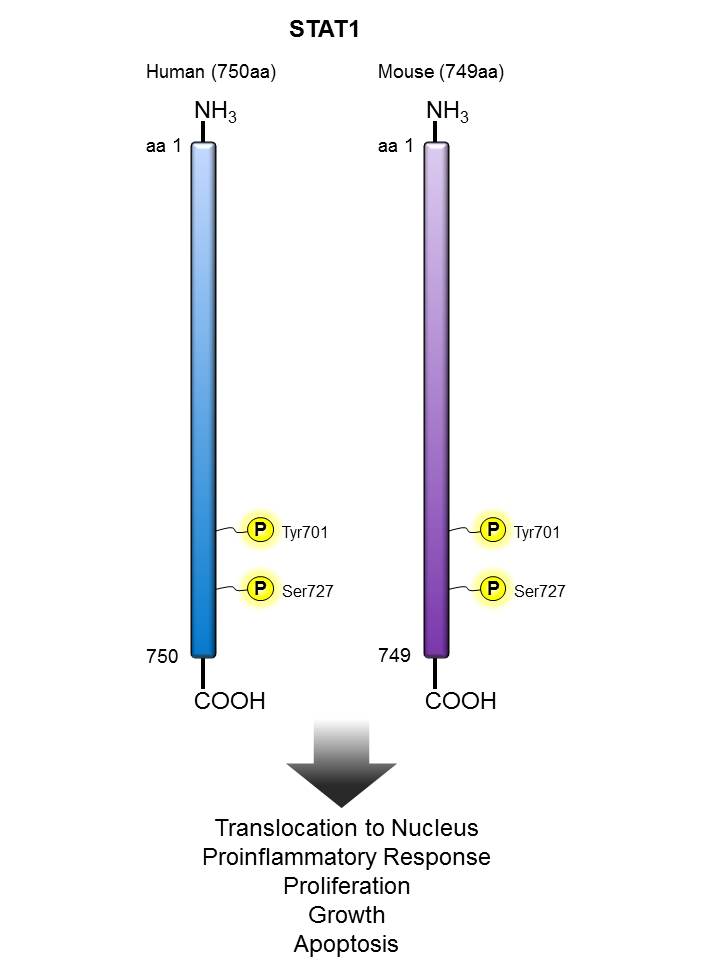
STAT1 |
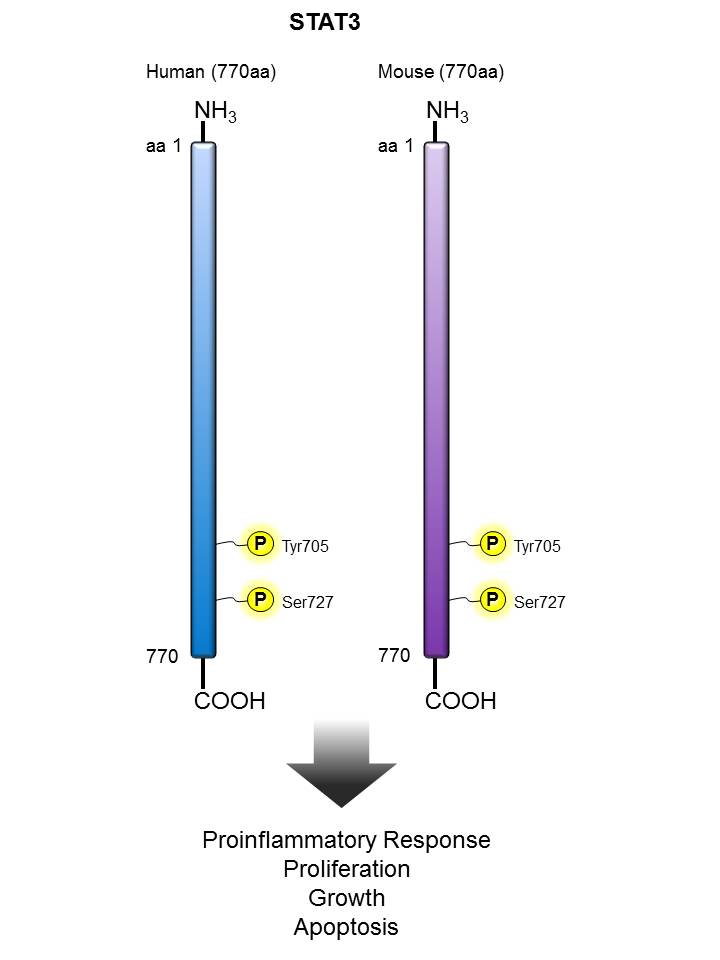
STAT3 |
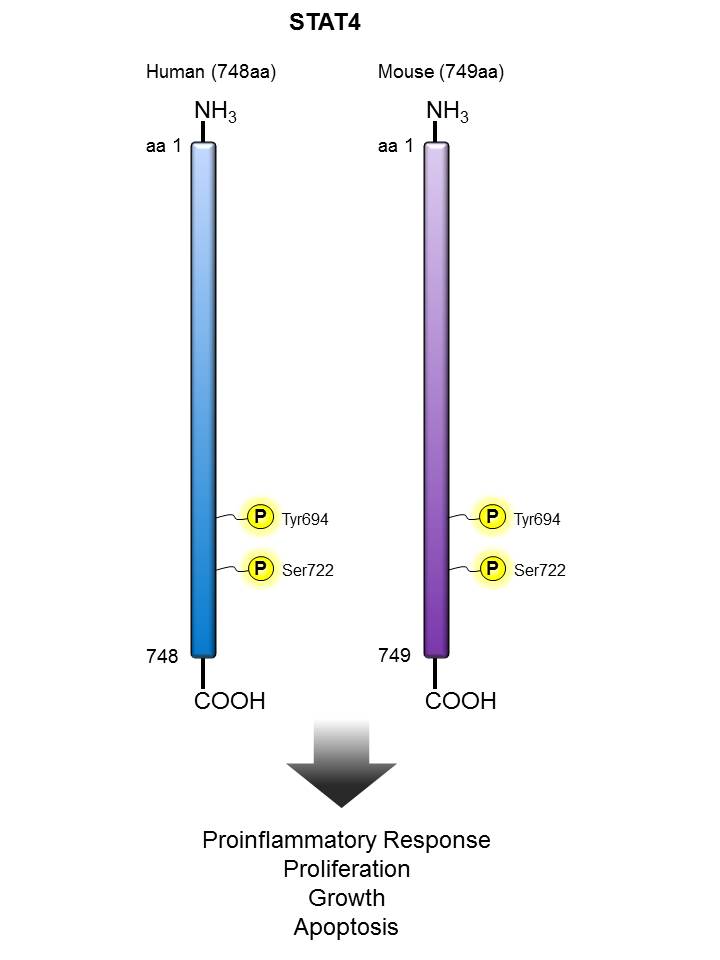
STAT4 |
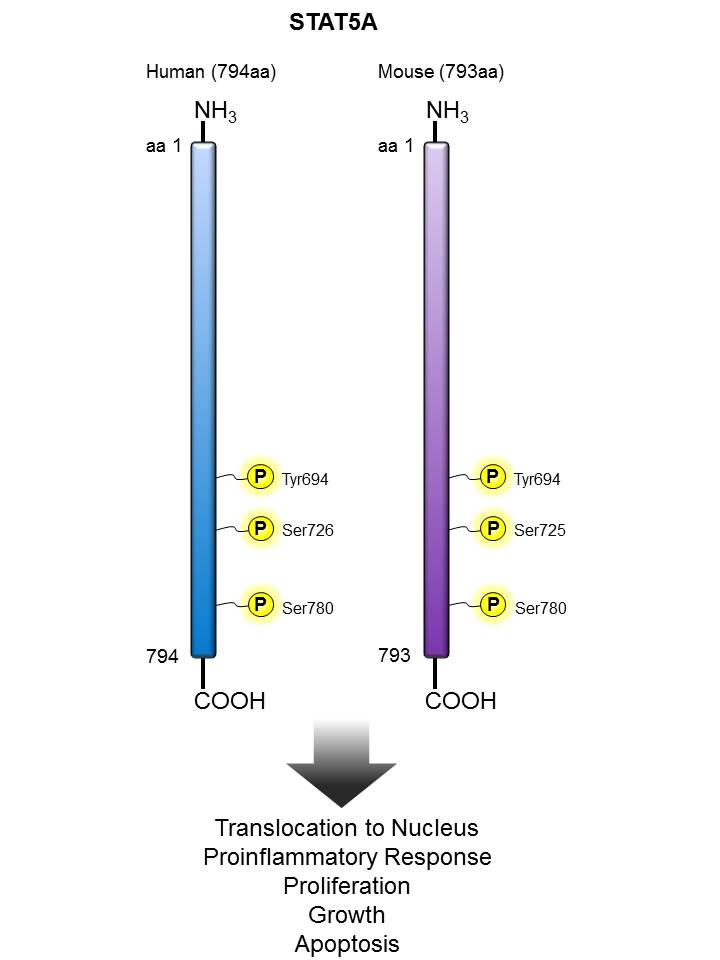
STAT5 |
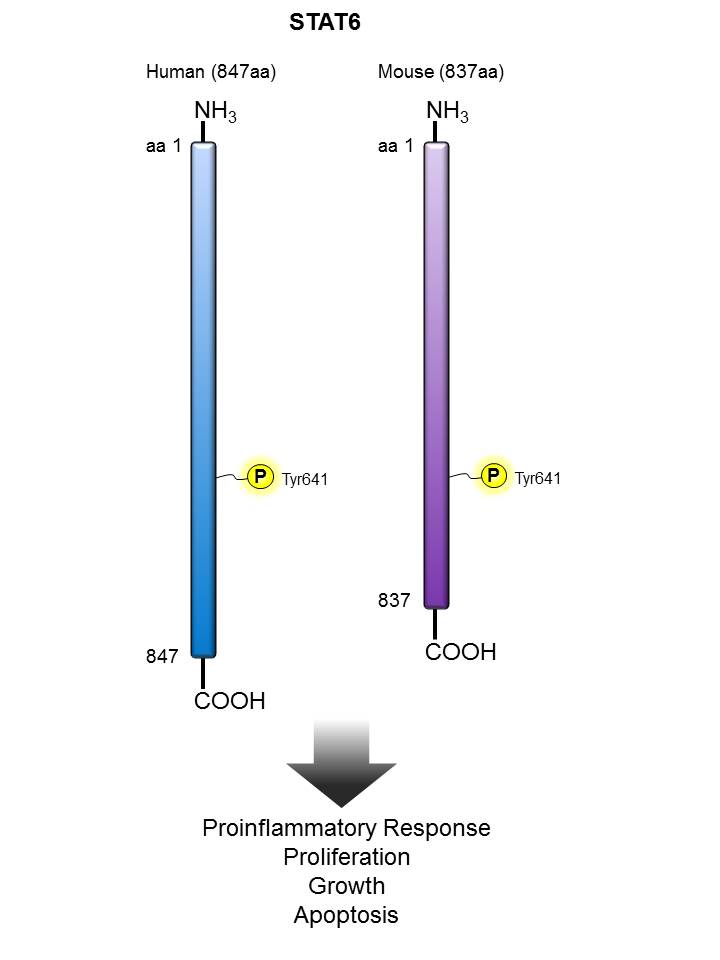
STAT6 |
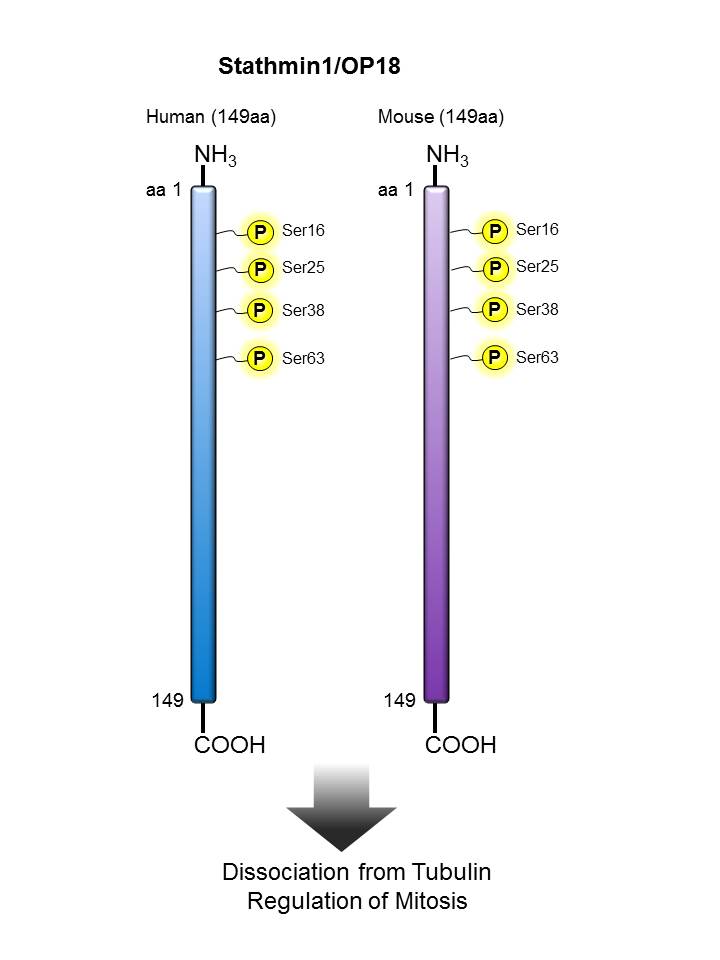
Stathmin/ OP18  |
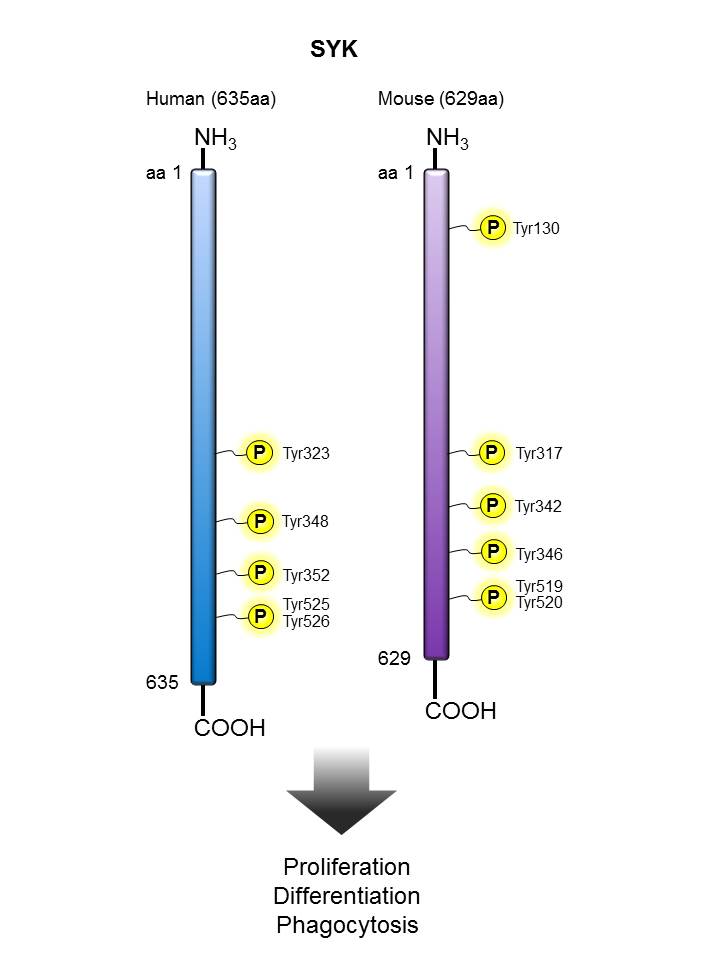
SYK |
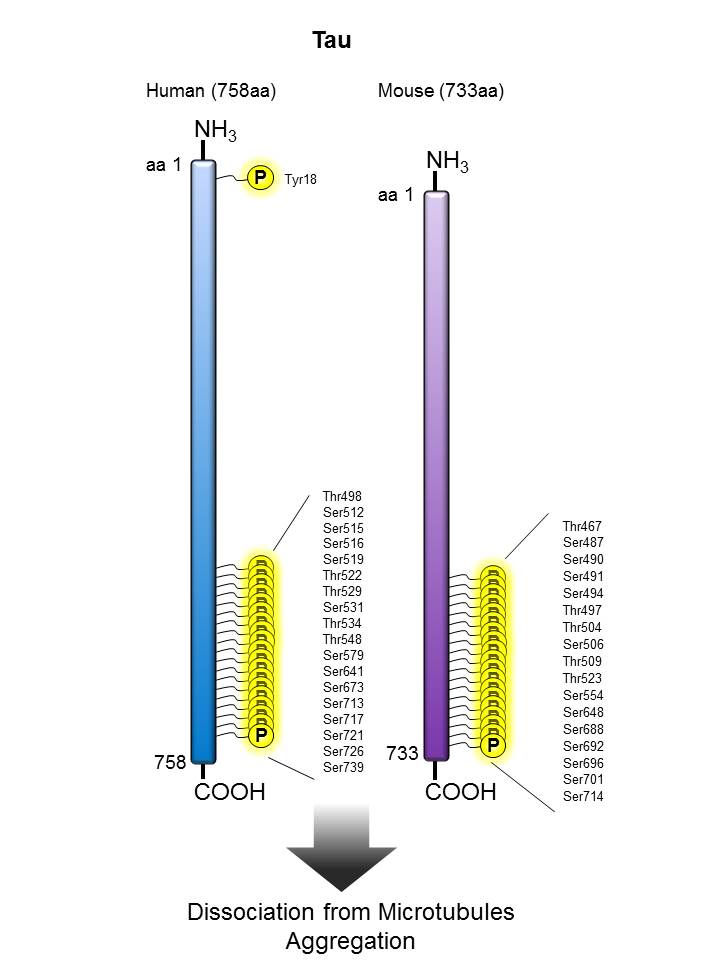
Tau |
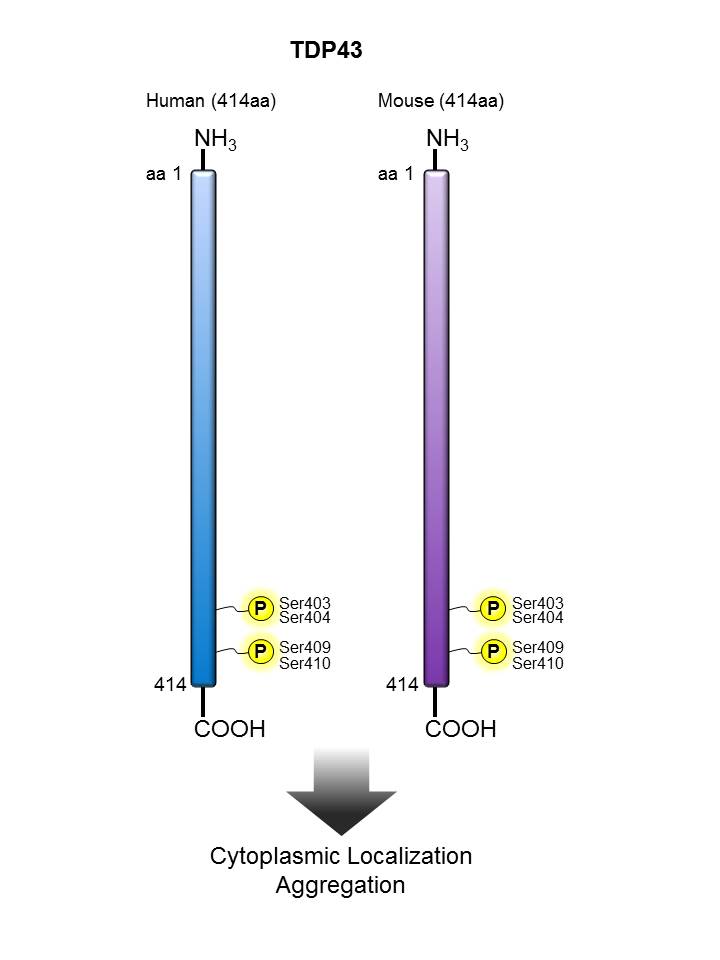
TDP43 |
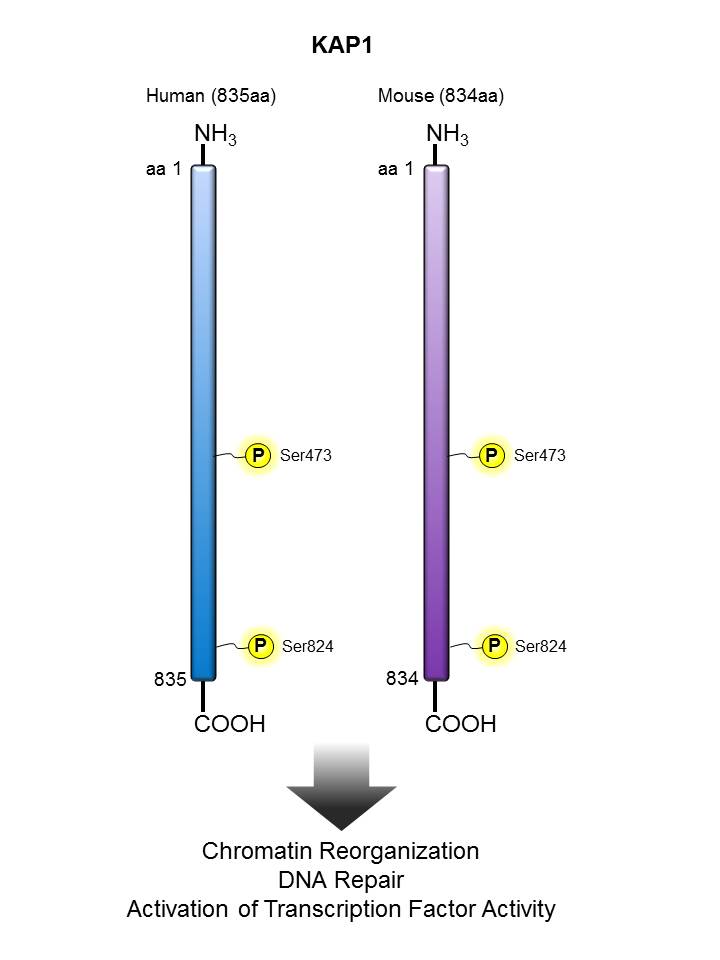
| TIF1β/ KAP1  |
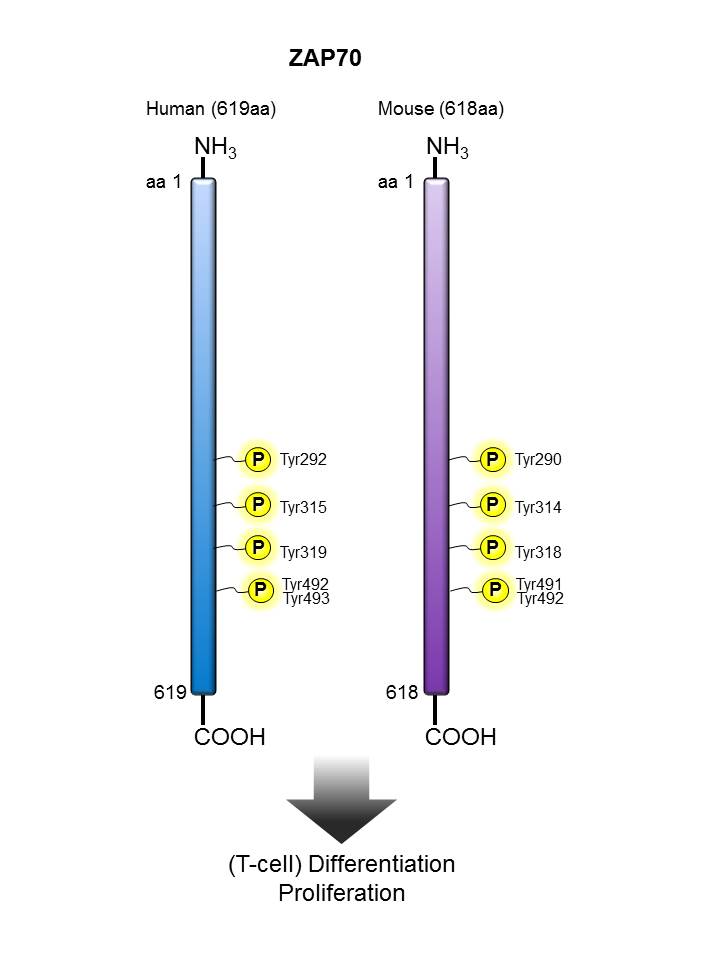
Zap70 |
|
We offer antibody clones raised against phosphorylated targets that have been validated for use in multiple experimental applications. For example, clone 4B11B69 raised against phosphorylated ERK1/2 (Tyr202/Tyr204) has been validated in-house for successful use in western blotting, immunofluorescence, and intracellular flow cytometry.
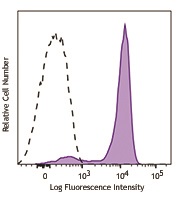
Human peripheral blood lymphocytes were stimulated with (filled histogram), or without (open histogram), Cell Activation Cocktail (without Brefeldin A) for 15 minutes, then fixed with Fixation Buffer, permeabilized with True-Phos™ Perm Buffer, and intracellularly stained with ERK1/2 Phospho (Thr202/Tyr204) (Clone 4B11B69) Alexa Fluor® 647.
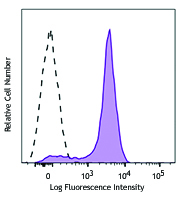
C57BL/6 mouse splenocytes were stimulated with (filled histogram) or without (open histogram) Cell Activation Cocktail (without Brefeldin A) for 15 minutes, then fixed with Fixation Buffer, permeabilized with True-Phos™ Perm Buffer, and intracellularly stained with ERK1/2 Phospho (Thr202/Tyr204) (Clone 4B11B69) Alexa Fluor® 647.
Superior Signal-to-Noise Ratio:
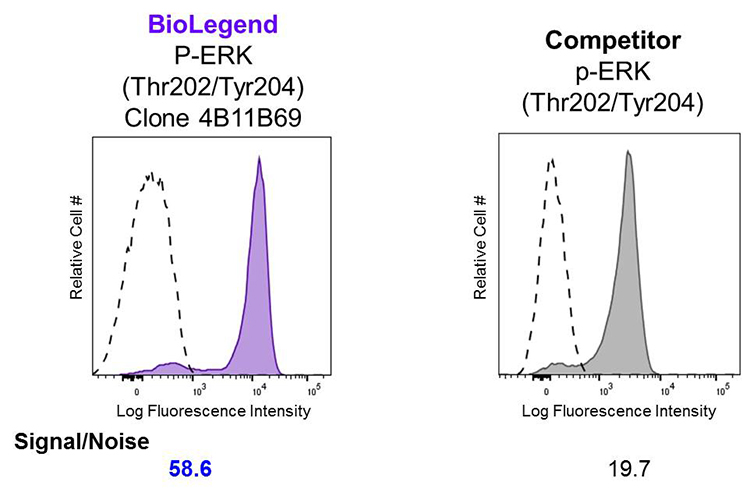
Human peripheral blood lymphocytes were stimulated with (filled histogram) or without (open histogram) Cell Activation Cocktail (without Brefeldin A) for 15 minutes, then fixed with Fixation Buffer, permeabilized with True-Phos™ Perm Buffer, and intracellularly stained with Alexa Fluor® 647 ERK1/2 Phospho (Thr202/Tyr204). Clone 4B11B69 was used at 0.125 µg/test and compared side-by-side against the competitor's product, which was used at 0.1 µg/test.
Western Blot (WB):
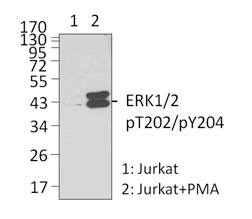
Left: Untreated (lane 1) and PMA-treated (lane 2) Jurkat whole cell lysates were resolved by electrophoresis, transferred to nitrocellulose, and probed with purified monoclonal anti-ERK1/2 Phospho (Thr202/Tyr204) (clone 4B11B69) antibody. Proteins were visualized using an anti-mouse-IgG secondary conjugated to HRP (Cat. No. 405306) and chemiluminescence detection.
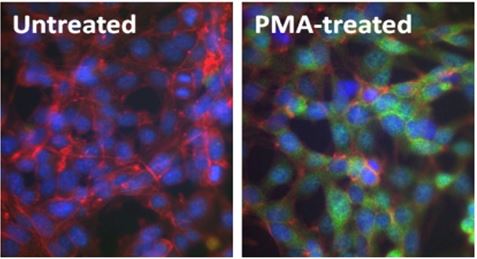
Immunofluorescence (IF):
Above: Untreated (left) and PMA-treated (20 nM PMA treatment for 10 minutes, right) mouse NIH-3T3 cells were stained with purified anti-ERK1/2 Phospho (Thr202/Tyr204) (clone 4B11B69) antibody, followed by staining with DyLight™ 488 conjugated goat anti-mouse IgG (green, Cat. No. 405310) antibody and Alexa Fluor® 594 conjugated phalloidin (red). Nuclei were stained with DAPI (blue).
Phospho-target Antibodies for Neuroscience
With the acquisition of Covance's antibody product portfolio, BioLegend now offers antibodies targeted towards phosphorylated proteins implicated in the biology of the brain, and neurodegenerative diseases.
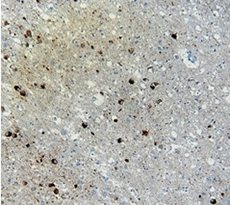
Immunohistochemistry (IHC):
(Left) IHC staining of anti-Tau Phospho (Thr181) antibody (clone M7004D06) on formalin-fixed paraffin-embedded Alzheimer's brain tissue. Following antigen retrieval using Sodium Citrate, the tissue was incubated with the primary antibody at 10 µg/ml for 60 minutes at room temperature. BioLegend's Ultra Streptavidin (USA) HRP Detection Kit was used for detection.
Western Blot (WB):
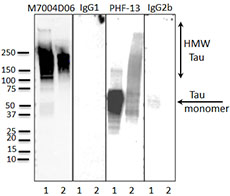
(Left) Western blot of anti-Tau Phospho (Thr181) (clone M7004D06), anti-Tau Phospho (Ser396) (clone PHF-13) antibodies, and isotype-matched control IgG1 and IgG2b. Lane 1: 20 µg of Parkinson’s disease human brain lysate; Lane 2: 2 µg of Tau paired helical filaments. Blots were incubated overnight at 4°C with 10 µg/ml of the primary antibody, followed by horseradish peroxidase labeled goat anti-mouse secondary antibody. Western blot of anti-Tau Phospho (Ser396) (clone PHF-13) serves as a positive control for Tau staining.
Kinases
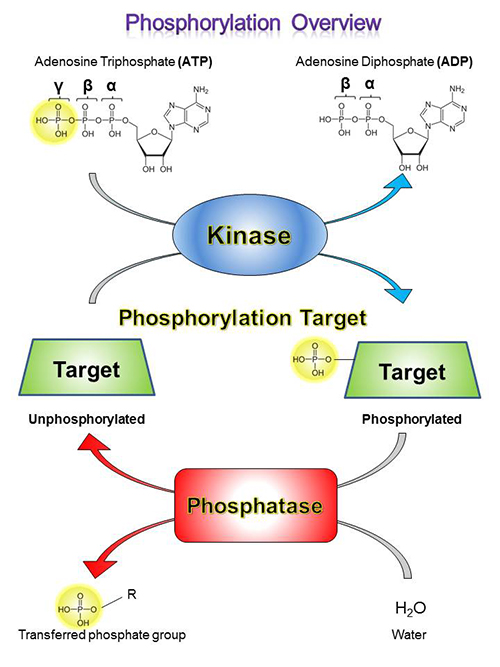
Kinases are enzymes that catalyze the addition of phosphoryl groups to certain amino acid residues on target molecules, which is called phosphorylation. To do this, a kinase will bind to ATP, hydrolyze the gamma (γ) phosphoryl, and bind to its target molecule, generating ADP in the process. Then, it transfers the phosphoryl group to the target molecule, dissociates, binds to a new ATP molecule. This process can then be repeated.
Phosphorylation Targets
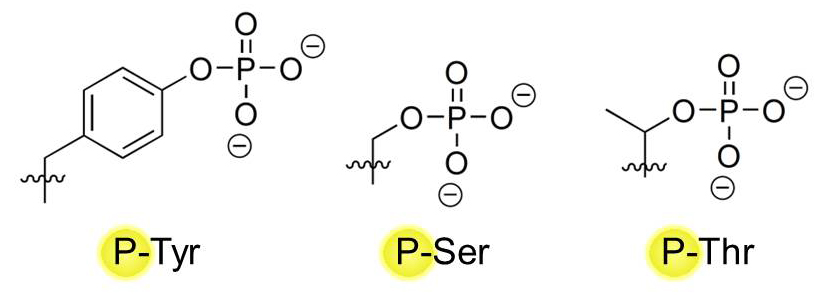
Proteins make up the majority of target molecules that can be phosphorylated by kinases, but lipids (e.g. Phosphatidylinositol-4,5-bisphosphate 3, or PI3) can also be phosphorylated. On proteins, three major amino acid residues serve as major sites of phosphorylation: Tyrosines (Tyr, Y) Serines (Ser, S), and Threonines (Thr, T). They all possess a hydroxyl (-OH) group in their variable chain that can serve as a placement site for a phosphate group by kinases:
Phosphatases
Phosphatases work in reverse to kinases, and are responsible for removing phosphate groups from their targets. Dephosphorylation that is catalyzed by phosphatases is important for maintaining homeostatic balance in the biological system – once a protein is phosphorylated for activation, it must be turned off via dephosphorylation by phosphatases or by protein degradation. Constitutively active phosphorylation signal pathways are hallmarks of numerous malignancies, including cancer.
True-Phos™ Perm Buffer
Finding the right conditions to stain for phosphorylated intracellular targets for flow cytometric analysis can be tricky. At BioLegend, we have developed the True-Phos™ Perm Buffer for flow cytometry, which is specifically designed to help detect our highly sensitive phosphorylation-specific antibodies. True-Phos™ Perm Buffer is not designed for use in western blotting or microscopy applications.
Representative Data:
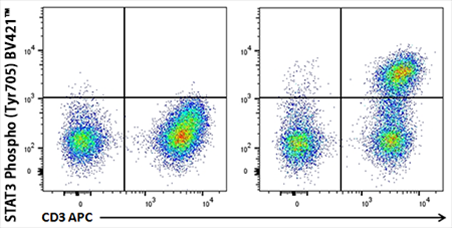
Human whole blood unstimulated (left panel) or stimulated with IL-6 for 15 minutes (right panel) were treated with RBC Lysis/Fixation Solution (10X), permeabilized with True-Phos™ Perm Buffer, and stained with CD3 APC and STAT3 Phospho (Tyr705) Brilliant Violet 421™

HeLa cells were treated with Nocodazole for 24 hours (right panel) or untreated (left panel), fixed with Fixation Buffer, permeabilized with True-Phos™ Perm Buffer, then stained with DAPI and Histone H3 Phospho (Ser10) Alexa Fluor® 647.
 Login / Register
Login / Register 






Follow Us