Fix Now? Fix Later? - Considerations for the Use of Paraformaldehyde Fixation in Flow Cytometry
Sometimes in the middle of a flow cytometry experiment, you have to fix your samples. There's a variety of reasons you'll need to fix samples including, but not limited to:
|
Despite all of these different reasons for you needing to fix your samples, have you considered factors, such as:
In this blog, we'll explore these questions related to fixation, and perhaps some food for thought for your next flow cytometry experiment when you run into a fixation dilemma. |
| For the purposes of this blog entry (more to come on the different types of fixatives...), we'll mainly focus on the use of paraformaldehyde/formaldehyde, commonly referred to as PFA, which is the most commonly used fixative in flow cytometry. PFA specifically refers to the solid, polymer form of formaldehyde (and dissolves into monomeric formaldehyde in aqueous solution), so what’s usually referred to canonically as "PFA fixation" is actually using the monomeric, formaldehyde solution. In this instance, PFA and formaldehyde are synonymous and interchangeable. A solution ranging from 1-4% PFA is typically used for fixation of samples for flow cytometry. In the case of sanitizing infectious samples, concentrations as low as 0.37% can effectively disinfect samples from HIV-infected patients1. Incubation for up to 45-60 minutes with 1% PFA, and 15-20 minutes with 4% PFA (e.g. BioLegend's buffer) is sufficient to fully fix the cells, and the cells can either be used for downstream processing (permeabilization for intracellular targets) or stored for future analysis at this stage. During a routine flow cytometry staining procedure that involves surface marker staining, there are several steps where you can decide to fix your samples - fix after surface marker staining, or fix before. In some instances, the decision for when you can fix is... fixed. For instance, if you're analyzing phospho-targets, binding of certain surface antigens by antibodies can alter intracellular signaling pathways shortly after contact, thus leading to an artifact in your phosphorylation results. In which case, you should fix the cells before the addition of the surface antibodies (which is why our recommended protocol to use for our True-Phos™ Perm Buffer to analyze intracellular phosphorylation targets is designed so that the fixation step precedes other procedures). Each has its own benefits and drawbacks, but here's a couple of things to watch out for in each instance. |
| Fixation BEFORE staining - epitope alteration: |
Due to the nature of fixatives, they can cause antigen epitope structures to be altered, which might render the antibodies unable to bind to their targets. Below are some examples of antibodies demonstrating loss of signal when stained on fixed cells: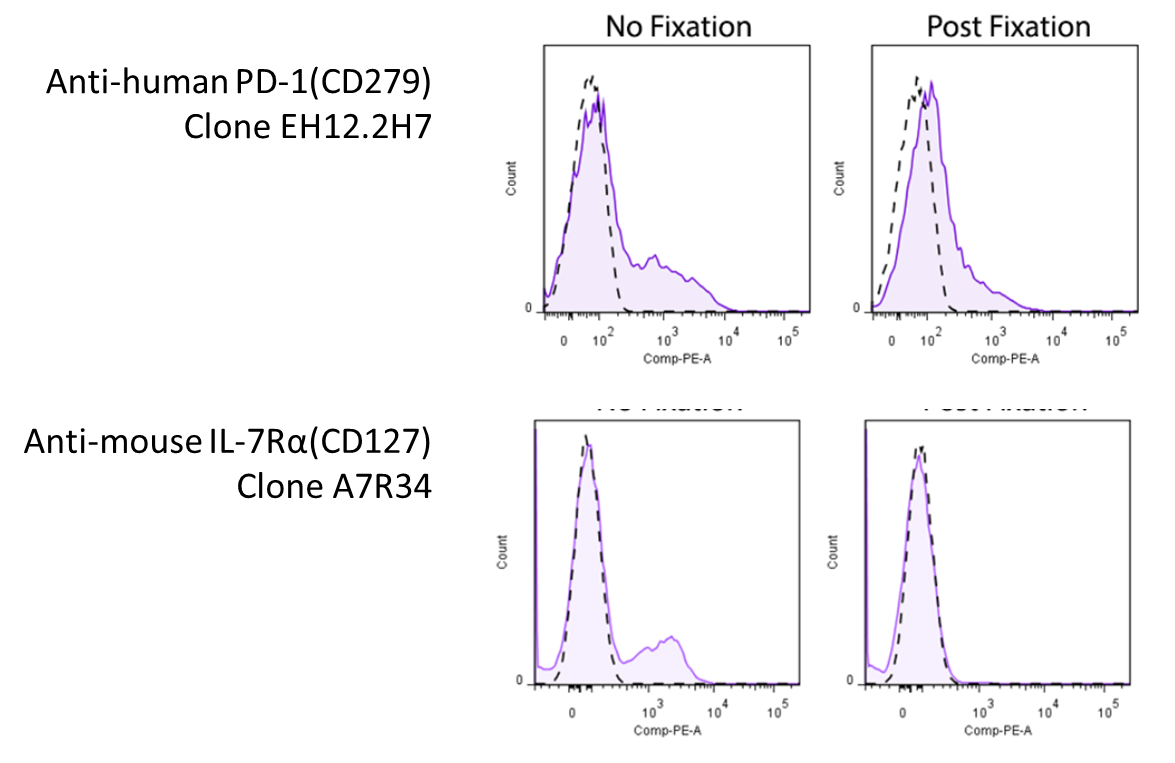 Representative plots for target cells stained with (Post-Fixation) or without 4% PFA fixation. Since this effect depends on the clone of antibody you're using, you will want to look around to see whether there's any information pertaining to the antibody you're using. However, you might be in luck! We've compiled a set of information and data (where available) on whether a particular antibody's binding is affected by 4% PFA fixation, which can be found on our Fixation webpage. For clones not listed on our site, you may want to consult the literature. |
| Fixation AFTER staining - fluorophore stability |
| Upon encountering fixative, fluorophores that are conjugated to the antibodies can potentially lose some of their signal. Protein-based dyes like PE and APC, and especially their tandems (i.e. PE/Cyanine7 and APC/Cyanine7), are more susceptible to the quenching of signal upon encountering fixative solutions, though in most cases they should be able to withstand a standard, 1-4% PFA fixation. However, harsher fixatives such as alcohols may be more detrimental to such fluors. In which case, we'd recommend leaning towards the use of synthetic dyes, such as Alexa Fluor® and Brilliant Violet™ (more specifically, BV421™ and BV510™) for your experiments, which will be able to withstand these types of fixation conditions more robustly, or consider staining after fixation. To combat the issue of tandem breakdown upon fixation, BioLegend has developed the FluoroFix™ Buffer - a PFA-based fixative buffer specially formulated to mitigate signal quenching by sensitive fluorophores. |
| So now that you've heard all about different considerations when it comes to fixing your samples for your flow cytometry experiments, it's time to get fixin'! Do you have any other questions regarding fixation procedures for your flow cytometry work? Any additional tips and considerations to add here? Let us know at tech@biolegend.com! |
| References: 1 Utility of formaldehyde fixation for flow cytometry and inactivation of the AIDS associated retrovirus Contributed by Kenta Yamamoto, PhD. |
 Login / Register
Login / Register 






Follow Us