Functional Antibodies
Our functional antibodies elucidate or affect the cellular functions of interest. These antibodies are quality-tested in-house for in vitro and in vivo functional assays such as blocking, neutralization, or activation. They are available in Low Endotoxin, Azide-Free (LEAF™) and Ultra-Low Endotoxin, Azide-Free (Ultra-LEAF™) formulations. Both are 0.2 µm filtered and eliminate unwanted effects from endotoxin and sodium azide (Table 1).
Among our selection of functional antibodies, GoInVivo™ products specialize in targeting immune checkpoint receptors, an important step in soluble factor-based immunotherapy to boost a patient’s own anti-tumor activity to fight cancer cells. GoInVivo™ products are all validated by a functional assay, formulated free of sodium azide, guaranteed pathogen-free based on PCR testing, and offered in bulk quantities (Table 1) at an exceptional price.
LEAF™, Ultra-LEAF™
- Quality-tested by functional assays for selected targets
- Formulated free of sodium azide
- 0.2 µm filtered to reduce endotoxin
GoInVivo™
- Specialized to target immune checkpoint receptors
- Validated by functional assay
- Formulated free of sodium azide
- Guaranteed pathogen-free based on PCR testing
- Offered in bulk quantities
Table 1.
|
Feature |
GoInVivo™ |
Ultra-LEAF™ |
LEAF™ |
|---|---|---|---|
|
Quality Tested by Flow Cytometry |
√ |
√ |
√ |
|
Free of Preservatives |
√ |
√ |
√ |
|
Custom Bulk Sizing |
√ |
√ |
√ |
|
Off-the-shelf Sizes |
5 mg – 1 g |
100 µg – 100 mg |
50 µg, 500 µg |
|
Endotoxin Levels |
< 1 EU/mg |
< 10 EU/mg |
< 100 EU/mg |
|
Functionally Validated* |
√ |
Some |
Some |
|
Pathogen Tested** |
√ |
|
|
* All GoInVivo™ antibodies are validated with in vitro functional assays, and in some cases, in vivo applications. Our Ultra-LEAF™ antibodies are functionally tested for selected targets only.
** All GoInVivo™ antibodies are pathogen free as tested by qPCR for up to 20 pathogens, including Mycoplasma.
GoInVivo™ products are tested to be free of the following pathogens based on the origin of the antibody. Pathogen-free is defined based on IDEXX BioResearch’s IMPACT test (PCR-based screening). For detailed information regarding the sensitivity of the assays please contact BioLegend Technical Service.
Mouse Antibodies: IMPACT™ 1. View testing profiles on the IDEXX site.
Rat Antibodies: IMPACT™ 5. View testing profiles on the IDEXX site.
Hamster Antibodies: IMPACT™ 7. View testing profiles on the IDEXX site.
Bulk Advantages
For some experiments, like animal models receiving in vivo antibody treatements, large quantities of antibodies can be required. To help researchers with these needs, we provide the ability to buy in large batches, which includes several benefits, including:
- Outstanding value
- Each lot is quality-tested and backed by our 100% guarantee
- Bulk orders provided in the Ultra-LEAF™ format (<0.01 EU/µg of protein)
- Rapid turnaround time
We also provide custom options for your functional antibodies:
- Bulk production from your hybridoma
- Custom concentrations available
- Custom endotoxin limits
- Custom formulation of purified or fluorophore-conjugated antibody
- Flexible packaging
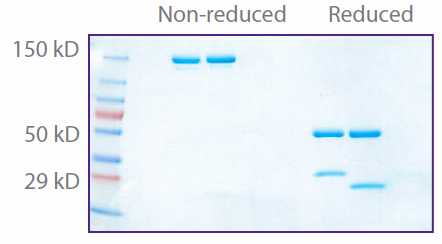
Purity determination: each lot of Purified and Ultra-LEAF™ Purified antibody is assessed for purity by SDS-PAGE.
Decipher the Complexity of Cancer With Functional Antibodies – B Cell Focus
Our expanding library of functional antibodies is designed to investigate the characteristics of a variety of cells. Here we specifically focus on our tools to accelerate your research on B cell cancers and B cell-related studies.
Anti-human CD20 (Rituximab Biosimilar, RUO) Recombinant Antibody
Our anti-human CD20 recombinant antibody (clone QA20A02) is a non-therapeutic biosimilar to Rituximab, a therapeutic antibody approved for the treatment of B cell malignancies and autoimmune disorders. Our Rituximab biosimilar is designed with the same variable regions as that of Rituximab, making it ideal for use in research and development of anti-tumor and immunosuppressive therapeutics.
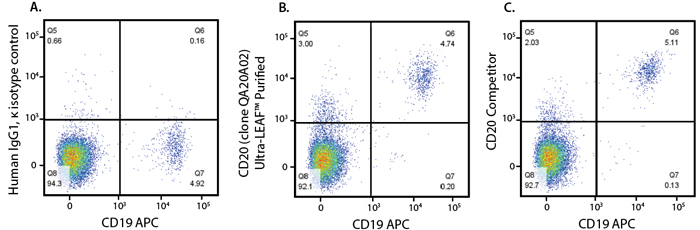
Human peripheral blood lymphocytes were stained with Ultra-LEAF human IgG1 isotype control (A), Ultra-LEAF Purified anti-human CD20 (Rituximab Biosimilar, RUO) Recombinant Antibody (clone QA20A02) (B), or anti-human CD20 (Rituximab biosimilar) from another supplier (C), followed by PE anti-human IgG Fc recombinant. Cells were co-stained with anti-human CD19.
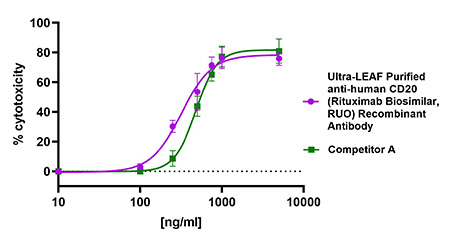
Ultra-LEAF Purified anti-human CD20 (Rituximab Biosimilar, RUO) Recombinant Antibody binds to human CD20 on the surface of Daudi cells and activates complement-dependent cytotoxicity (CDC) in a dose-dependent manner similar to another Rituximab Biosimilar from a competitor (green). This antibody has an IC50 range of 200-500 ng/mL.
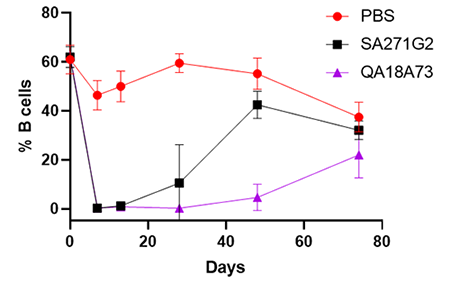
Ultra-LEAF™ Purified anti-mouse CD20 Recombinant Antibody (clone QA18A73)
Our anti-mouse CD20 recombinant antibody (clone QA18A73) was designed with a mouse isotype for increased in vivo stability and delayed clearance. It shows more sustained B cell depletion over our previous clone, SA271G2.
C57BL/6 mice were retro-orbitally injected with 200 µg of Ultra-LEAF™ purified anti-mouse CD20 (clone SA271G2, black line, N=4), Ultra-LEAF™ purified anti-mouse CD20 recombinant (clone QA18A73, purple line, N=4), or vehicle only (PBS, red line, N=4). Peripheral blood was analyzed at indicated intervals, and average % B cells calculated for each group (measured as % CD19+B220+ cells).
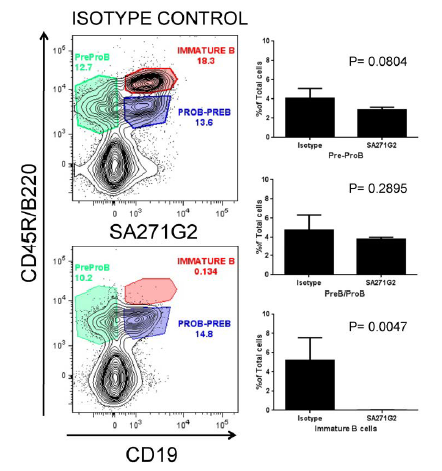
Ultra-LEAF™ Purified anti-mouse CD20 Antibody (clone SA271G2)
Our validation studies show how effectively in vivo administration of monoclonal anti-mouse antibody (clone SA271G2) depletes only the immature B cell subset from bone marrow, but not pre-pro, pro, or pre-B cells.
In vivo administration of mAb SA271G2 depletes immature stage of bone marrow B cells. Bone marrow cells from SA271G2-treated mice or the isotype-treated control group were stained to identify the different B cell subsets. Only the immature B cells (CD19hi/B220hi) were depleted. Data shown was gated on the lymphoid population.
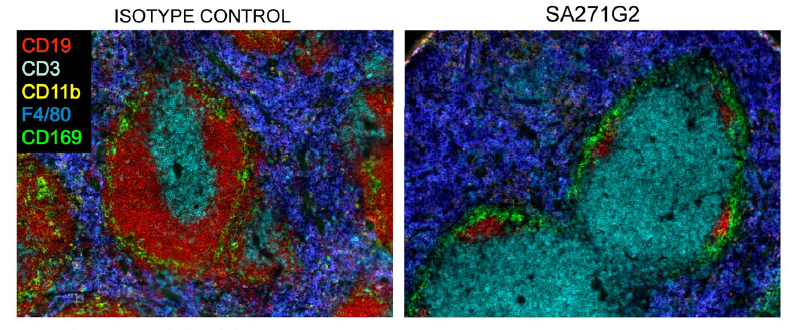
Likewise, treatment with mAb SA271G2 only depletes the CD20+ cells, without any effect on T cells, macrophages, or other cell types. Moreover, tissue integrity of the spleen was maintained despite the almost complete absence of B cells.
C57BL/6 mice were injected intravenously with 250 μg of Ultra-LEAF™ Purified anti-mouse CD20 (clone SA271G2) (right) or Ultra-LEAF™ Purified rat IgG2b, isotype control (left). At day 7, the spleens were collected, sectioned, and stained for CD19 (red), CD3 (cyan), CD169 (green), CD11b (yellow), and F4/80 (blue) in microscopy analysis.
 Login / Register
Login / Register 






Follow Us