MojoSort™ Whole Blood Human CD8 Nanobeads Protocol
Reagent List
|
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator; the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Protocol Steps
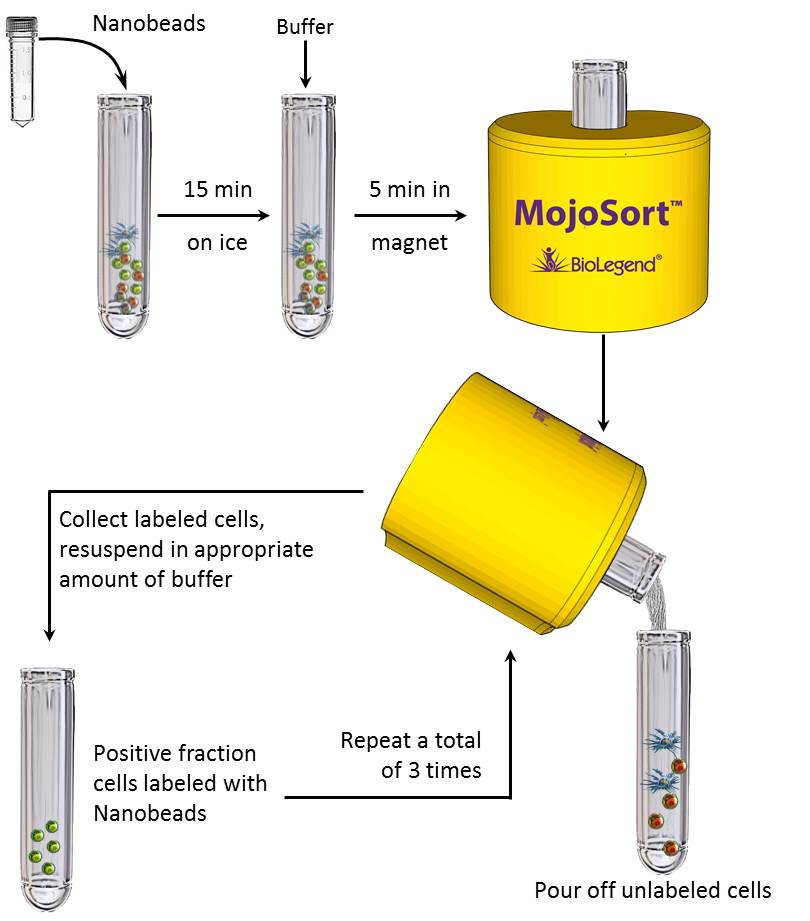
Product description and procedure summary:
The cells targeted by the Nanobeads are either selected or depleted by incubating your sample with the directly conjugated magnetic particles. The magnetically labeled fraction is retained by the use of a magnetic separator. After collection of the targeted cells, downstream applications include functional assays, gene expression, phenotypic characterization, etc.
Note: This procedure is optimized using 1mL whole blood per tube. Scale volumes in this procedure accordingly, e.g. using 2 mL whole blood, use twice the amount of nanobeads. Prepare fresh MojoSort™ Buffer solution by diluting the 5X concentrate with sterile distilled water. Scale up volumes if using 14 mL tubes and Magnet, and place the tube in the magnet for 10 minutes.
Protocol
- Collect whole blood in collection tube that has anticoagulant, preferably EDTA.
Note: Keep MojoSort™ Buffer on ice throughout the procedure. - Add 1mL of whole blood into a new tube.
- Resuspend the beads by vortexing, maximum speed, 5 touches. Add 50 µL of Antibody Nanobeads. Mix well and incubate on ice for 15 minutes. Scale up the volume accordingly if separating more volume of whole blood. For example, add 100 µL of Nanobeads for separating 2 mL of whole blood.
- Add MojoSort™ Buffer, see Table 1 for volume.
Note: If you observe aggregates, filter the suspension. To maximize yield, you can disrupt the aggregates by pipetting the solution up and down.
Table 1. MojoSort™ Whole Blood Human CD8 Nanobeads Protocol: Separation Volumes and Magnet Times
Catalog No. MojoSort™ Buffer Volume Magnetic Incubation Time MojoSort™ magnet 480019 For samples ≤ 1 mL:
Add 3mL buffer for separation5 minutes MojoSort™ magnet 14mL 480020 For samples ranging 1 – 5 mL:
Top volume up to 10mL for each separation
(e.g. for 5mL sample add 5mL buffer for the first separation, then add 10mL buffer for subsequent separations)10 minutes
- Place the tube in the magnet, see Table 1 for magnetic incubation time.
Optional: Take a small aliquot before placing the tube in the magnet to monitor purity and yield. Keep unused cells to be used as control or other applications if needed. - Pour out the unlabeled fraction. If these are your cells of interest, DO NOT DISCARD. Resuspend the labeled cells in MojoSort™ Buffer.
- Repeat steps 4-6 on the labeled fraction twice more for a total of 3 separations. Pool the unlabeled fractions and keep the labeled cells. The fraction that is not of interest may be useful as staining controls, to monitor purity/yield, or other purposes.
Optional: Take a small aliquot to monitor purity and yield. - Resuspend the magnetically labeled cells in desired volume, making sure to rinse the sides of the tube for better recovery.
- (Optional) Lyse remaining erythrocytes using Red Blood Cell Lysis Buffer (10X Cat. No. 420301 / 420302).
Chart Protocol:
Application notes: To use this product in magnetic separation columns, a titration of the Biotin-Antibody Cocktail and Nanobeads should be performed. Optimal concentration for magnetic separation columns is lot-specific. Please contact BioLegend Technical Service (tech@biolegend.com) for further assistance on how to use MojoSort™ Nanobeads in magnetic separation columns.
 Login / Register
Login / Register 







Follow Us