The following protocol describes cell surface protein staining with TotalSeq-A antibodies, with or without TotalSeq-A hashtag antibodies. This protocol should serve as a guide for the general staining of cell surface proteins with TotalSeq-A antibodies that can be used prior to single-cell partitioning with the platform and/or a community-verified single-cell protocol of your choice. This protocol has been optimized using liquid, single-antibody TotalSeq-A conjugates, and TotalSeq-A cocktails (Universal and TBNK).
Read through this protocol in its entirety and the associated user guides for the single-cell platform, or community-verified single-cell protocol you intend to use prior to starting so you can plan accordingly.
Note:
User is solely responsible for determining whether there are any licenses or other intellectual property rights necessary for user’s intended use of any BioLegend products or any protocol or with any other products or technologies and assumes all risk and liability arising from such use.
Commonly Used Abbreviations and Terminology:
- ADT – Antibody Derived Tag. Refers to a TotalSeq DNA-barcoded oligonucleotide that is directly conjugated to a specific antibody clone of interest. Each antibody clone was raised against a specific extracellular protein epitope and can be used to characterize the expression of that surface antigen on cells. Thus, ADTs serve as DNA tags used to catalog and quantify distinct surface protein expression levels.
- HTO – Hash Tag Oligonucleotide. Refers to a TotalSeq DNA-Barcoded Oligonucleotide that is directly conjugated to a cocktail of two independent antibody clones that are specific for surface proteins known to be ubiquitously expressed on various cell types. The intention of an HTO is to enable researchers to load multiple samples into a single reaction and maintain the ability to determine sample origin.
- CITE-Seq – Cellular Indexing of the Transcriptome and Epitopes by Sequencing. This application was first described by Stoeckius et al. (Nat Methods 14, 865–868 (2017)) of the NY Genome Center as they used antibodies coupled with oligonucleotides to simultaneously measure proteins and RNA at a single-cell level.
- Cell Hashing – Application where oligo-tagged antibodies against ubiquitously expressed surface proteins uniquely label cells from distinct samples, which can be subsequently pooled. By sequencing these tags alongside the cellular transcriptome, users can assign each cell to its original sample.
Explore our TotalSeq Resources and Learning webpage to discover technical resources and publications that showcase the utilization of TotalSeq-A antibodies.
Protocol Overview
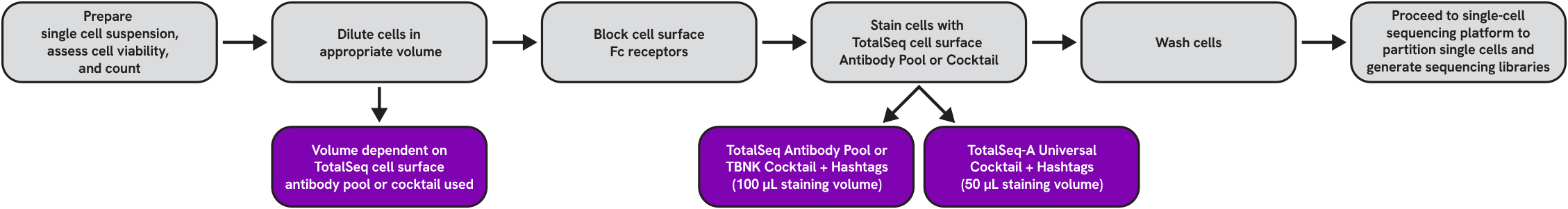
Reagent and Consumable List
- Cell Staining Buffer (BioLegend, Cat. No. 420201)
- Human TruStain FcX™ (Fc Receptor Blocking Solution) (BioLegend, Cat. No. 422301)
- TruStain FcX™ PLUS (anti-mouse CD16/32) (BioLegend, Cat. No. 156603)
- Low Protein Binding Microcentrifuge Tubes (ThermoFisher Scientific, Cat. No. 90410 or equivalent)
- 12 x 75 mm Falcon™ Round-Bottom Polystyrene Tubes (Fisher Scientific, Cat. No. 14-959-1A or equivalent)
Additional Suggested Reagents
- TotalSeq™-A Antibodies
- TotalSeq™-A Human Universal Cocktail, V1.0, (BioLegend, Cat No. 399907)
- TotalSeq™-A Human TBNK Cocktail, (BioLegend, Cat. No. 399901)
- TotalSeq™-A Mouse Universal Cocktail, V1.0, (BioLegend, Cat. No. 199901)
Best Practices and Important Considerations for Best Results
Surface Staining
Cell washing
- When washing cells, it is extremely important to thoroughly decant the wash buffer, and, upon the addition of new wash buffer, that the cell pellet is resuspended either with pipette mixing or gentle vortexing. When decanting, pour off the wash buffer in a single firm, but not forceful motion. Following decanting, continue to hold the tube inverted and remove the remaining droplets on the lip of the tube by gently dabbing with a clean paper towel before returning the tube to an upright position. This technique should be used during all cell washes.
Optimal cell staining with TotalSeq™ antibodies
- Liquid single-antibody TotalSeq conjugates require optimization of staining concentration (titration) to obtain the best results, as performed by the end user of the antibodies. Optimization of antibody staining concentration is essential to obtaining good quality data in antibody-based applications such as surface staining using TotalSeq antibodies.
- Antibody titration for sequencing-based applications using TotalSeq antibodies is best performed using matching procedures as much as possible. This means titration-by-sequencing if possible. In the absence of titration-by-sequencing, it may be possible to replicate this protocol using fluorescent antibodies of the same clone and titrate via flow cytometry. For more information regarding titration of TotalSeq-A antibodies, please read Tips and Tricks for Titrating TotalSeq Antibodies. BioLegend can provide recommended concentration ranges for most TotalSeq antibodies, and these can be obtained by contacting BioLegend Technical Services.
- Throughout this protocol, the term “antibody pool” is defined as a user-created antibody pool of titrated single TotalSeq antibodies.
- BioLegend’s lyophilized Human TotalSeq-A antibody cocktails have been validated for use with this application. The cocktails are optimized for specific staining reaction sizes. The TotalSeq-A Human TBNK Cocktail is optimized to stain 1 x 106 cells in 100 µL staining volume, and the TotalSeq-A Human Universal Cocktail V1.0 has been optimized to stain 5 x 105 cells in 50 µL staining volume.
Note:
We have demonstrated staining of up to 2 x 106 cells from PBMCs with 1 test of the TotalSeq™-A Human TBNK and Universal Cocktail, V1.0 with no effect on staining efficiency allowing for reliable detection of lineage markers using this protocol. Sample specific variations on cocktail performance are expected with larger cell input numbers and customers should verify adequate sensitivity in cocktail performance in their experiment. It is important that final staining volume, regardless of cell number used, not exceed 100 µL for the TBNK cocktail and 50 µL for the Universal Cocktail.
Protocol
Cell Surface Staining
1. Prepare cell suspension with preferred or recommended method
Note:
- This protocol has been optimized using fresh human PBMCs isolated using density gradient centrifugation. Whole blood or lysed whole blood is not recommended. If using cells isolated with a different procedure, users may need to verify the antibody staining pattern using alternative methods.
- BioLegend has not tested this protocol using single-cell suspensions derived from enzymatically digested tissue. Enzymatic digestion may result in alterations of surface protein epitopes and impact staining with TotalSeq antibodies. Optimization of staining conditions and concentrations may be required.
2. Count and assess cell viability
2.1 Using your preferred method, carefully count all cells to ensure accurate quantitation and assess cell viability.
Note:
Contact BioLegend Technical Services with any questions regarding cell viability. Ideal cell viability is >95%. Low cell viability is associated with poor single-cell sequencing data. If low cell viability is observed, users may need to enrich live cells or repeat cell suspension preparation.
3. Dilute cells in an appropriate volume prior to staining
3.1 When using an antibody pool or the TotalSeq-A TBNK Cocktail:
3.1.1 If working with human cells, dilute 1 x 106 cells in 45 μL of Cell Staining Buffer in a 12 x 75 mm flow cytometry tube.
3.1.2 If working with mouse cells, dilute 1 x 106 cells in 49.5 μL of Cell Staining Buffer in a 12 x 75 mm flow cytometry tube.
3.2 When using a TotalSeq-A Universal Cocktail:
3.2.1 If working with human cells, dilute 5 x 105 cells in 22.5 μL of Cell Staining Buffer in a 12 x 75 mm flow cytometry tube.
3.2.2 If working with mouse cells, dilute 5 x 105 cells in 24.75 μL of Cell Staining Buffer in a 12 x 75 mm flow cytometry tube.
4. Fc receptor blocking
4.1 If using an antibody pool or the TotalSeq-A TBNK Cocktail:
4.1.1 For human cells, add 5 μL of Human TruStain FcX to 1 x 106 cells in 45 µL of Cell Staining Buffer (total volume = 50 μL).
4.1.2 For mouse cells, add 0.5 µL of TruStain FcX PLUS (anti-mouse CD16/32) blocking reagent to 1 x 106 cells in 49.5 µL of Cell Staining Buffer (total volume = 50 μL).
4.2 If using the TotalSeq-A Universal Cocktail:
4.2.1 For human cells, add 2.5 μL of Human TruStain FcX Fc blocking reagent to 5 x 105 cells in 22.5 µL of Cell Staining Buffer (total volume = 25 μL).
4.2.2 For mouse cells, add 0.25 μL of TruStain FcX PLUS (anti-mouse CD16/32) blocking reagent to 5 x 105 cells in 24.75 µL of Cell Staining Buffer (total volume = 25 μL).
4.3 Incubate for 10 minutes at 4°C.
4.4 While cells are incubating in Fc Block, proceed to step 5 – Stain cells with cell surface antibodies.
5. Stain cells with cell surface antibodies
Note:
If you plan to multiplex samples using TotalSeq hashtags, there are two commonly used approaches to staining samples with hashtag antibodies.
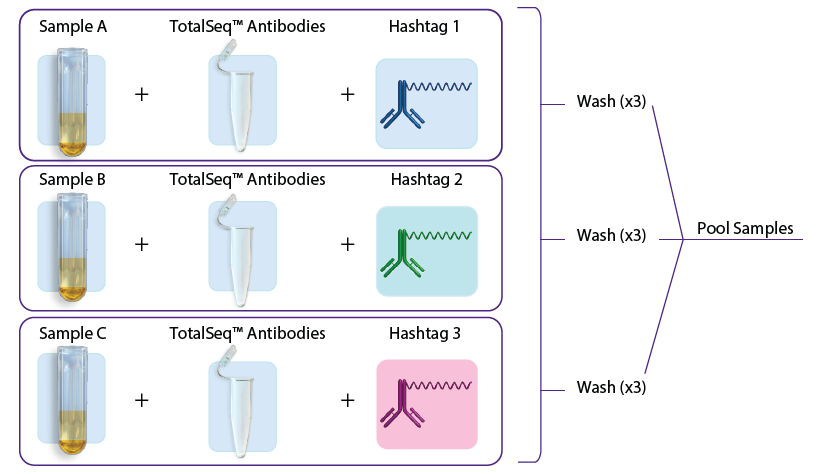
- In the first approach, individual samples are stained with TotalSeq antibodies (user-created antibody pool or with our pre-optimized TotalSeq-A cocktails) and hashtags in a single step, washed three times to remove unbound antibodies, and subsequently pooled for loading into one or more single-cell sequencing reactions. See figure 1A.
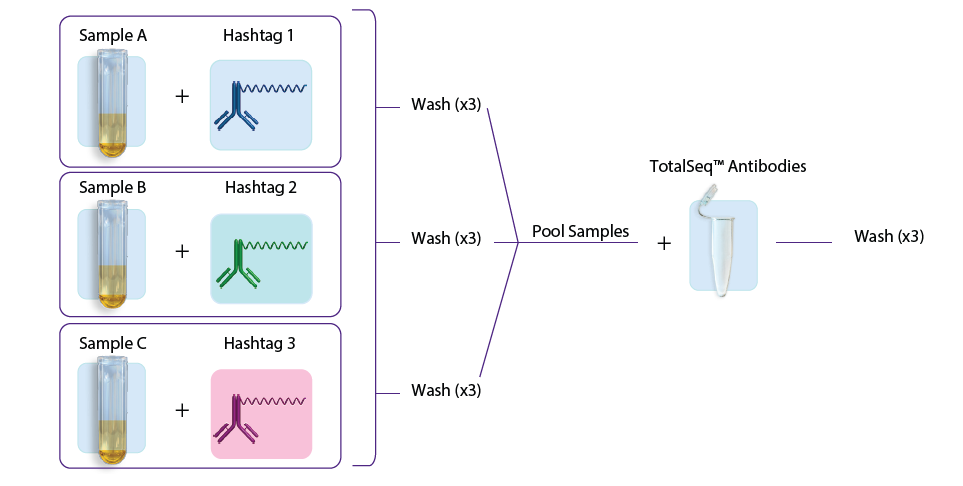
- The second approach involves first staining individual samples with hashtags, washing three times, sample pooling, and then staining with the TotalSeq antibodies (user-created antibody pool or with our pre-optimized TotalSeq-A cocktails), followed by another three washes. See figure 1B.
- Whenever possible, we recommend the first approach above as the second approach can result in increased sample loss and reduced cell viability. Reducing sample washing will result in higher background and poor data quality. Additional information regarding use of TotalSeq hashtag antibodies can be found here – Efficient Multiplexing With TotalSeq™ Hashtags.
- For assistance in de-multiplexing after sequencing, please contact BioLegend Technical Services.
A.
B.
Figure 1: A. Staining with TotalSeq antibodies and hashtags in a single step before pooling samples. B. Staining individual samples with hashtag reagents followed by pooling and staining with additional TotalSeq antibodies in subsequent steps.
5.1 Staining individual samples with TotalSeq™ Hashtag Antibodies for sample pooling
Note:
- If you are not using TotalSeq Hashtags, or if you are staining cells simultaneously with an antibody pool or one of our TotalSeq- A cocktails and hashtags in a single step before pooling samples (figure 1A), proceed to step 5.2.
- If sequentially staining individual samples with hashtag antibodies, pooling samples, and then staining with a TotalSeq-A antibody pool or one of our TotalSeq-A cocktails as seen in figure 1B, follow the steps below before staining your samples with an antibody pool or cocktail. Please note that when sequentially staining, cell recovery may be diminished, and cell viability may be negatively impacted in some cases.
5.1.1 Make a unique hashtag staining solution for each sample using a titrated amount of each hashtag in Cell Staining Buffer. Add the calculated amount of each hashtag antibody to a low protein binding microcentrifuge tube, and bring up the total volume with Cell Staining buffer to 50 µL if using an antibody pool or TotalSeq-A TBNK cocktail. Or, bring the total volume up to 25 µL if using the TotalSeq-A Universal Cocktail.
5.1.2 Centrifuge the hashtag solution at 14,000 x g at 2 – 8°C for 10 minutes before adding to the cells to ensure the removal of protein aggregates.
5.1.3 Carefully pipette out the prepared hashtag solution, avoiding the bottom of the tube, and add it to the 50 µL or 25 µL blocked cell suspension.
5.1.4 Incubate for 30 minutes at 4°C.
5.1.5 Wash cells, add 3 mL of Cell Staining Buffer, mix by gently pipetting 5 times. Centrifuge at 4°C for 5 minutes at 400 – 600 x g depending on your sample type.
5.1.6 Repeat wash twice for a total of 3 washes.
Note:
It is extremely important to thoroughly decant the wash buffer and resuspend the cell pellet either with pipetting or gentle vortexing. Discard supernatant with a single firm, but not forceful motion. Proceed to absorb any remaining liquid on the lip of the tube with a clean paper towel.
5.1.7 After the final wash, resuspend each sample in 100 µL of Cell Staining Buffer, and verify cell concentration for each sample.
5.1.8 Based on each sample’s cell concentration, combine an equal number of cells from each sample to achieve 1 x 106 cells if using an antibody pool or the TotalSeq-A TBNK Cocktail, or 5 x 105 cells if using a TotalSeq-A Universal Cocktail in a low protein binding microcentrifuge tube. The final volume will be dependent on the specific staining reaction size of the antibody pool or cocktail being used.
- If staining cells with an antibody pool or the TotalSeq-A TBNK cocktail and the volume of combined cells is less than 50 µL, adjust volume with Cell Staining Buffer up to 50 µL to achieve 1 x 106 cells/50 µL. If the final volume of combined cells is greater than 50 µL, centrifuge at 4°C for 5 minutes at 400 – 600 x g. Following centrifugation, adjust the volume to 50 µL by removing the Cell Staining Buffer. After addition or removal of the Cell Staining Buffer, gently resuspend cells by pipetting.
- If staining cells with the TotalSeq-A Universal cocktail and the volume of combined cells is less than 25 µL, adjust volume with Cell Staining Buffer up to 25 µL to achieve 5 x 105 cells/25 µL. If the final volume of combined cells is greater than 25 µL, centrifuge at 4°C for 5 minutes at 400 – 600 x g. Following centrifugation, adjust the volume to 25 µL by removing the Cell Staining Buffer. After addition or removal of the Cell Staining Buffer, gently resuspend cells by pipetting.
Examples:
- If you combined 6 samples at 10 µL each for a total of 60 µL and used an antibody pool or TotalSeq-A TBNK Cocktail, you would remove 10 µL after centrifugation for a total volume of 50 µL, resulting in 1 x 106 cells/50 µL.
- If you combined 4 samples at 10 µL each for a total of 40 µL and used a TotalSeq-A Universal Cocktail to stain cells, you would remove 15 µL of buffer after centrifugation for a total of 25 µL, resulting in 5 x 105 cells/25 µL.
5.1.9 Proceed with staining your combined samples using either the Cell Staining with TotalSeq with Antibody Pool section 5.2.1, or Cell Staining with TotalSeq-A Universal Cocktail or TotalSeq-A TBNK Cocktail section protocols below. Cells will already be stained with TotalSeq Hashtag Antibodies; do not add additional Hashtag Antibodies to the TotalSeq-A antigen-specific antibody pool.
5.2 Cell staining with TotalSeq™ Antibody Pool and TotalSeq™ Hashtags
5.2.1 Prepare the antibody pool by combining titrated amounts of each specific TotalSeq-A antibody in a low protein binding microcentrifuge tube. For more information regarding titration of TotalSeq-A antibodies, please read Tips and Tricks for Titrating TotalSeq Antibodies. If you have additional questions, please reach out to BioLegend Technical Services.
5.2.2 When performing dual staining with TotalSeq-A cell hashtag antibodies and TotalSeq-A antigen-specific antibodies, we recommend adding cell hashing antibodies into each respective sample’s TotalSeq-A antibody pool (figure 1A).
5.2.3 If the antibody pool volume is less than 50 µL, adjust the volume with Cell Staining Buffer up to 50 µL. If the volume of the pool is above 50 µL, no volume adjustment is necessary.
5.2.4 Centrifuge the antibody pool at 14,000 x g at 2 – 8°C for 10 minutes before adding to the cells. This is critical to ensure the removal of protein aggregates.
5.2.5 Carefully pipette out the prepared antibody pool, avoiding the bottom of the tube, and add the TotalSeq antibody pool to the 50 µL blocked cell suspension.
5.2.6 Incubate for 30 minutes at 4°C.
5.3 Cell staining with TotalSeq-A Universal or TotalSeq-A TBNK Cocktail and TotalSeq Hashtags
5.3.1 If staining cells with a lyophilized TotalSeq-A Universal or TBNK Cocktail, follow the reconstitution steps below.
5.3.2 Equilibrate the lyophilized panel vial(s) to room temperature for 5 minutes.
5.3.3 Place the lyophilized panel vial in an empty microcentrifuge tube, and spin down at 10,000 x g for 30 seconds at room temperature.
5.3.4 If cell hashing samples simultaneously with the TotalSeq-A Universal or TBNK Cocktail, please refer to the “Spike-In” Guidance protocol prior to reconstitution of the lyophilized cocktail to determine how to make the Cell Staining Buffer + spike-in reconstitution mix. Then proceed to the next step, 5.3.5, to reconstitute the lyophilized cocktail. If not hashing or if your samples are already stained with hashtags, proceed to the next step, 5.3.5.
5.3.5 If using the TotalSeq-A Universal Cocktail, rehydrate by adding 27.5 µL of Cell Staining Buffer or the Cell Staining Buffer + hashtag mix. If using the TBNK cocktail, rehydrate by adding 50 µL of Cell Staining Buffer or the Cell Staining Buffer + hashtag mix. Replace the cap and vortex for 10 seconds.
5.3.6 Incubate at room temperature for 5 minutes.
5.3.7 Vortex again and spin down at 10,000 x g for 30 seconds at room temperature.
5.3.8 Transfer the entire volume, 27.5 µL for the TotalSeq-A Universal Cocktail, or 50 µL for the TotalSeq-A TBNK Cocktail, to a low protein binding microcentrifuge tube.
5.3.9 Centrifuge at 14,000 x g for 10 min at 4°C. Important: Centrifugation of the reconstituted cocktail at 14,000 x g at 2 – 8°C for 10 minutes is critical to ensure the removal of antibody aggregates.
5.3.10 Transfer 25 µL or 50 µL of the reconstituted cocktail you used to the tube containing 25 µL or 50 µL of FcR-blocked cells, taking care to avoid the bottom of the tube. The final staining volume will be 50 µL for cells stained with a TotalSeq-A Universal Cocktail, or 100 µL for cells stained with the TotalSeq-A TBNK Cocktail. Mix by gently pipetting 5 times.
5.3.11 Incubate for 30 minutes at 4°C.
6. Wash cells
6.1 Add 3 mL of Cell Staining Buffer and mix by gently pipetting 5 times. Centrifuge at 4°C for 5 minutes at 400 – 600 x g depending on your sample type. Repeat this step twice for a total of 3 washes.
Note:
It is important to thoroughly decant the wash buffer and resuspend the cell pellet by either pipetting or gentle vortexing. Discard supernatant with a single firm, but not overly forceful motion. Proceed to absorb any remaining liquid on the lip of the tube with a clean paper towel.
6.2 After the final wash, decant the supernatant and resuspend cells in 200 µL of Cell Staining Buffer for an approximate final volume of 250 – 350 µL. Gently mix the cells by pipetting.
6.3 Slowly filter cells through 40 µm Flowmi™ Cell Strainer into a low protein binding microcentrifuge tube.
Note:
40 µm Flowmi™ Cell Strainer may be too small for some sample types.
6.4 Count cells and assess cell viability.
6.5 Adjust cell concentration using PBS according to the input requirements of your single-cell partitioning platform.
Recommended sequencing depth for Cell Surface Protein libraries
To obtain sufficient read coverage for Cell Surface Protein libraries, follow the recommended library loading and pooling specifications provided by the single-cell partitioning platform of your choice. See the table below for BioLegend sequencing depth recommendations for Cell Surface Protein libraries.
|
Library Type |
Minimum Sequencing Depth |
|---|---|
|
Cell Surface and Intracellular Protein Library <100 Antibody Derived Tag (ADT) panel |
5,000 |
|
Cell Surface and Intracellular Protein Library ≥100 ADT panel |
10,000 |
 Login / Register
Login / Register 






Follow Us