Advanced flow cytometry requires advanced tools. BioLegend’s Fire tandem fluorophores push the limits of your flow capabilities by expanding into spectral spaces previously unused in conventional cytometry. In addition, we also offer Fire dyes with enhanced stability and brightness properties for existing channels. Ignite your next discovery with these phycobiliprotein (PE, APC) and PerCP-based dyes, specifically designed for spectral cytometry and other advanced flow cytometry applications.
Fire Dyes
PerCP/Fire™ 780
PerCP/Fire™ 780 expands blue laser options for multicolor flow cytometry panels. It is excited by the 488 nm blue laser and emits maximally at 774 nm. It can be used on conventional cytometers with a 780/60 filter, but is optimal for spectral instruments with both blue and yellow/green lasers. This allows it to be unmixed from dyes like PE/Cyanine7 and PerCP/Fire™ 806. The strong brightness of this fluorophore makes it ideal to be paired with antigens with low expression levels.
Excitation and Emission Spectra of PerCP/Fire™ 780
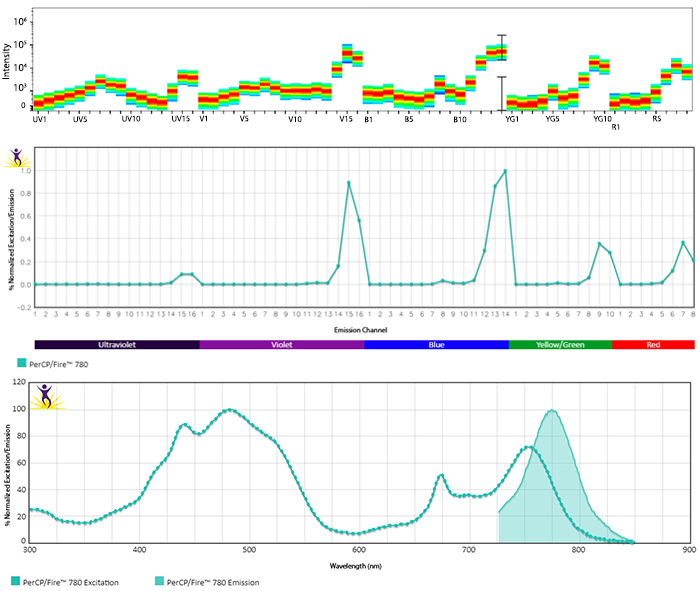
Emission spectra (top) and normalized emission spectra (middle) of PerCP/Fire™ 780 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PerCP/Fire™ 780 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PerCP/Fire™ 780 obtained from a spectrophotometer. To compare PerCP/Fire™ 780 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Multicolor Compatibility of PerCP/Fire™ 780
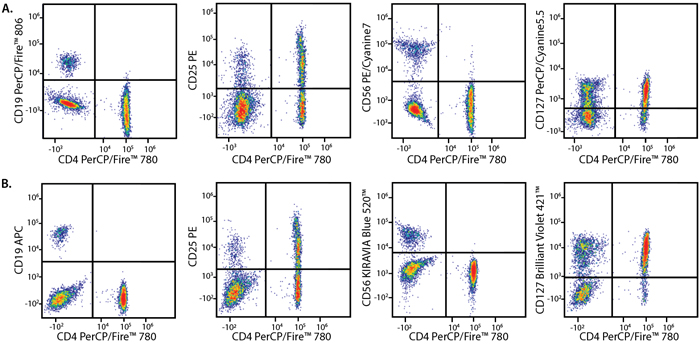
Panel A:
|
Marker |
Fluorophore |
|---|---|
|
CD4 |
PerCP/Fire™ 780 |
|
CD19 |
PerCP/Fire™ 806 |
|
CD25 |
PE |
|
CD56 |
PE/Cyanine7 |
|
CD127 |
PerCP/Cyanine5.5 |
Panel B:
|
Marker |
Fluorophore |
|---|---|
|
CD4 |
PerCP/Fire™ 780 |
|
CD19 |
APC |
|
CD25 |
PE |
|
CD56 |
KIRAVIA Blue 520™ |
|
CD127 |
BV421™ |
PBMCs were stained with a multicolor panel with either a high (Panel A) or low (Panel B) degree of spectral complexity and overlap. Samples were analyzed on a 5-laser Cytek® Aurora cytometer.
PerCP/Fire™ 780 Stability
Photostability
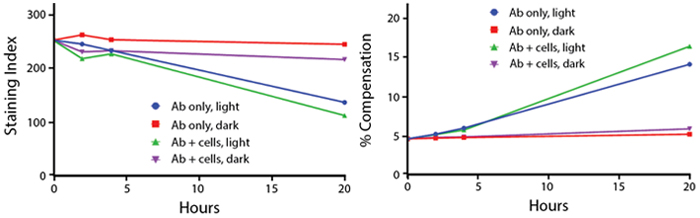
Anti-human CD4 (clone SK3) PerCP/Fire™ 780 was stored in clear vials (Ab only) and left exposed to light or protected in the dark, as indicated. Antibodies were stored for the indicated time points and then used to stain PBMCs. Samples labeled "Ab + cells" contain human PBMCs stained with anti-human CD4 PerCP/Fire™ 780. Stained cells were then left in the light or protected, as indicated.
Stability in Fixatives
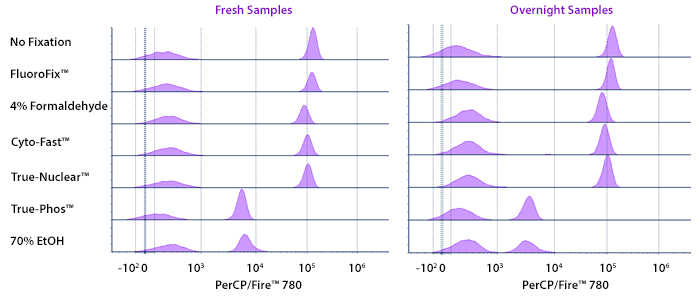
Human PBMCs were stained with anti-human CD4 (clone SK3) conjugated to PerCP/Fire™ 780 and fixed using the respective protocols for each buffer set. Fresh samples were fixed and read on a 5-laser Cytek® Aurora Cytometer immediately. Overnight samples were fixed and stored overnight in Cyto-Last™ Buffer before reading.
Heat Stability
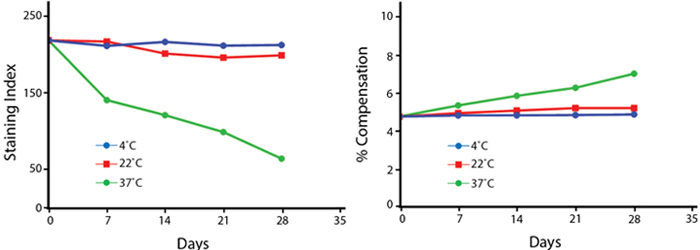
Anti-human CD4 (clone SK3) PerCP/Fire™ 780 was aliquoted and incubated at the indicated temperatures over the course of 28 days. The antibodies were then used to stain human lysed whole blood from a single donor.
Note: Percent compensation for each experiment was obtained on a 5-laser Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
PerCP/Fire™ 806
PerCP/Fire™ 806 is an innovative and unique flow cytometry fluorophore excited by the 488 nm blue laser that emits beyond 800 nm. It can be used on conventional cytometers with a 780/60 filter, but is optimal for spectral instruments with both blue and yellow/green lasers. This allows it to be unmixed from dyes like PE/Cyanine7 and PE/Fire™ 810. Its potent brightness makes it suitable for antigens with low expression levels.
Excitation and Emission Spectra of PerCP/Fire™ 806
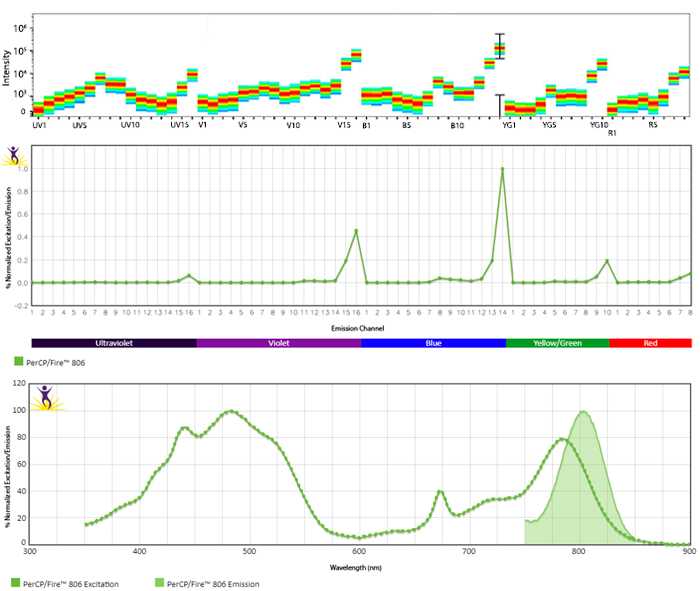
Emission spectra (top) and normalized emission spectra (middle) of PerCP/Fire™ 806 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PerCP/Fire™ 806 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PerCP/Fire™ 806 obtained from a spectrophotometer. To compare PerCP/Fire™ 806 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Multicolor Compatibility of PerCP/Fire™ 806
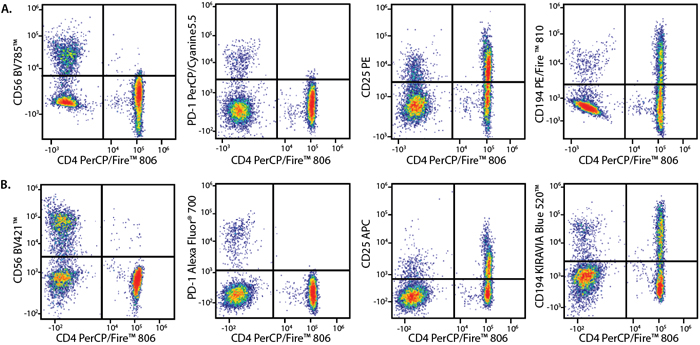
Panel A:
|
Marker |
Fluorophore |
|---|---|
|
CD4 |
PerCP/Fire™ 806 |
|
CD25 |
PE |
|
CD56 |
BV785™ |
|
CD194 |
PE/Fire™ 810 |
|
PD-1 |
PerCP/Cyanine5.5 |
Panel B:
|
Marker |
Fluorophore |
|---|---|
|
CD4 |
PerCP/Fire™ 806 |
|
CD25 |
APC |
|
CD56 |
BV421™ |
|
CD194 |
KIRAVIA Blue 520™ |
|
PD-1 |
Alexa Fluor® 700 |
RBC-lysed and washed human blood lymphocytes were stained with a multicolor panel with either a high (Panel A) or low (Panel B) degree of spectral complexity and overlap.
PerCP/Fire™ 806 Stability
Photostability
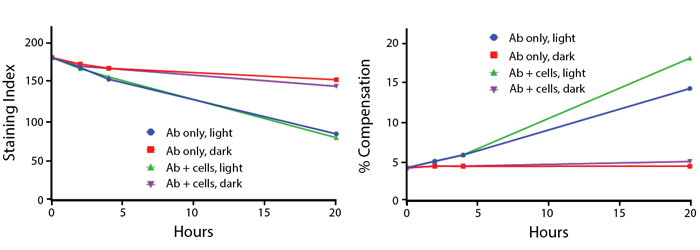
Anti-human CD4 (clone SK3) PerCP/Fire™ 806 was stored in a clear vial (Ab only) and left exposed to light or protected in the dark, as indicated. Antibodies were stored for the indicated time points and then used to stain PBMCs. Samples labeled "Ab + cells" contain human PBMCs stained with anti-human CD4 PerCP/Fire™ 806. Stained cells were then left in the light or protected, as indicated.
Stability in Fixatives
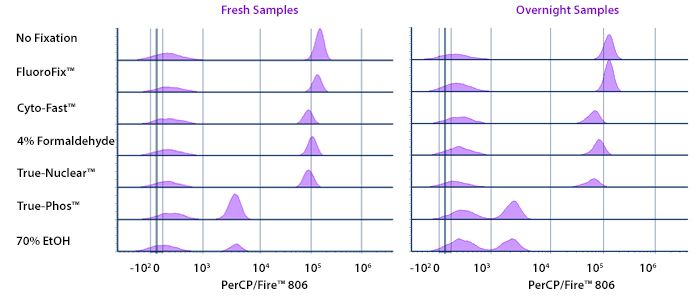
Human PBMCs were stained with anti-human CD4 (clone SK3) conjugated to PerCP/Fire™ 806 and fixed using the respective protocols for each buffer set. Fresh samples were fixed and read on a 5-laser Cytek™ Aurora Cytometer immediately. Overnight samples were fixed and stored overnight in Cyto-Last™ Buffer before reading.
Heat Stability
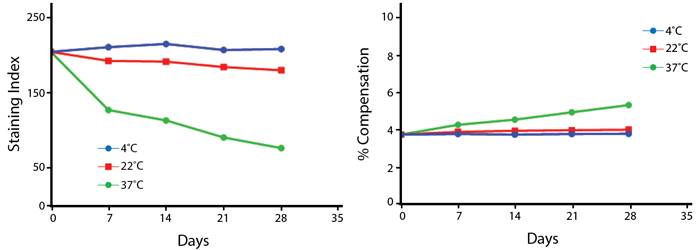
Anti-human CD4 (clone SK3) PerCP/Fire™ 806 was aliquoted and incubated at the indicated temperatures over the course of 28 days. The antibodies were then used to stain human lysed whole blood from a single donor.
Note: Percent compensation for each experiment was obtained on a 5-laser Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
APC/Fire™ 750
APC/Fire™ 750 was BioLegend’s first Fire tandem dye, originally developed to provide a more temperature and photostable alternative to APC/Cyanine7. APC/Fire™ 750 also has lower compensation requirements than APC/Cyanine7 conjugates while maintaining an equal level of brightness. Additionally, APC/Fire™ 750 has minimal non-specific binding to monocytes, as has been observed with APC/Cyanine7. Lastly, APC/Fire™ 750 is equivalent to or brighter than APC-H7 in all conjugates tested.
Excitation and Emission Spectra of APC/Fire™ 750
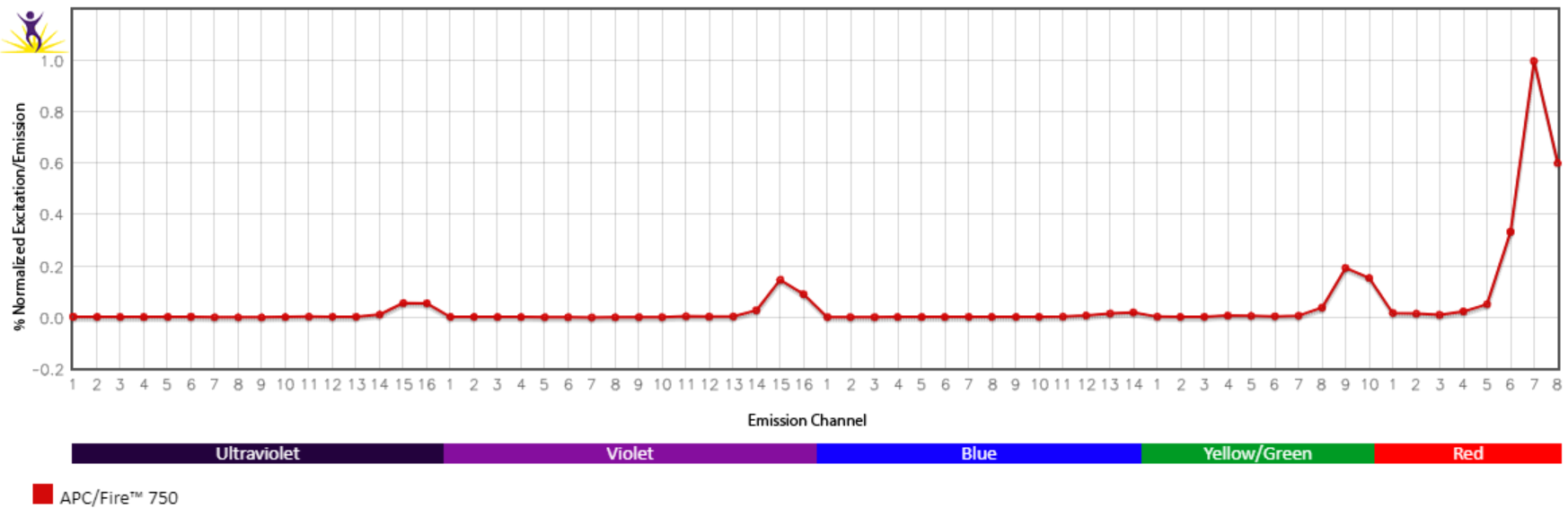
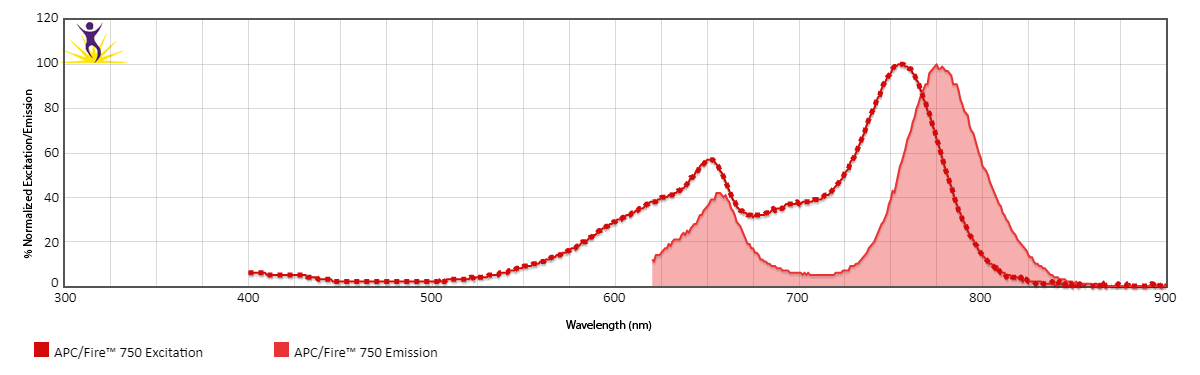
Emission spectra (top) and normalized emission spectra (middle) of APC/Fire™ 750 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare APC/Fire™ 750 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra of APC/Fire™ 750 obtained from a spectrophotometer (bottom). To compare APC/Fire™ 750 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
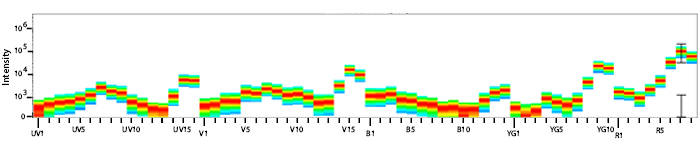
Spectral Spillover of APC/Fire™ 750
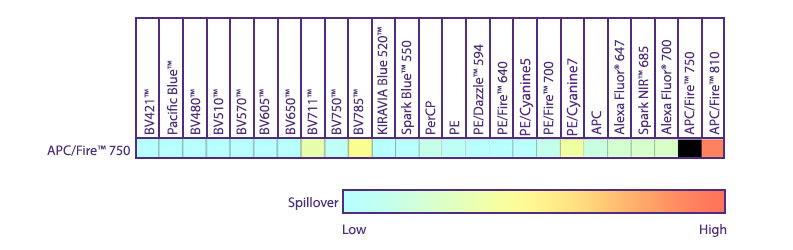
Spillover impact of APC/Fire™ 750 into detection channels of a 5-laser Cytek® Aurora Spectral Cytometer.
Spectral Compatibility with Zombie NIR™ in Spectral Flow Cytometry
Unlike comparable APC tandem dyes like APC/Cyanine7 and others, APC/Fire™ 750 has advantageous spectral properties that allow it to be unmixed from the fixable viability dye Zombie NIR™ in spectral applications. This gives the APC/Fire™ 750 and Zombie NIR™ combination special utility on spectral flow cytometers, allowing the user to conveniently slot both a viability probe and antibody stain into a narrow region of detection.
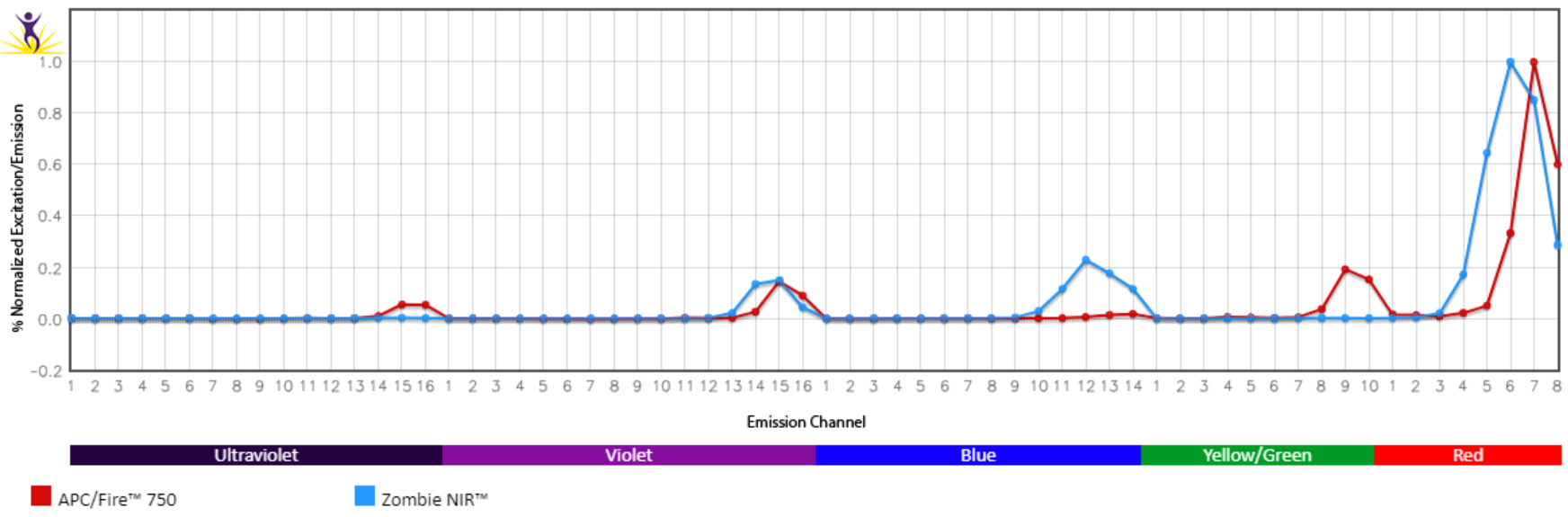
Normalized emission spectra of APC/Fire™ 750 and Zombie NIR™ as run on a 5-laser Cytek® Aurora Spectral Cytometer.
Multicolor Compatibility of APC/Fire™ 750
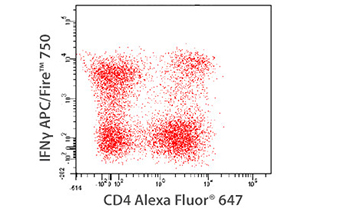 PMA+Ionomycin (6 hours, in the presence of Monensin) stimulated human PBMCs were stained with anti-CD4 Alexa Fluor® 647 and then treated with Fixation Buffer followed by permeabilization with 1X Intracellular Staining Permeabilization Wash Buffer and stained with anti-IFN-γ APC/Fire™ 750 for 30 min.
PMA+Ionomycin (6 hours, in the presence of Monensin) stimulated human PBMCs were stained with anti-CD4 Alexa Fluor® 647 and then treated with Fixation Buffer followed by permeabilization with 1X Intracellular Staining Permeabilization Wash Buffer and stained with anti-IFN-γ APC/Fire™ 750 for 30 min.
Brightness Comparison
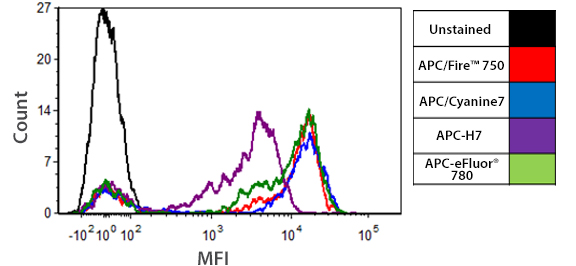 Human whole blood was stained for 20 min with anti-CD3 conjugates of APC/Fire™ 750, APC/Cyanine7, APC-H7, or APC-eFluor® 780 at each manufacturer's recommended optimal dilution, followed by RBC lysis and wash steps. Histograms were gated on lymphocyte populations based on forward and side scatter.
Human whole blood was stained for 20 min with anti-CD3 conjugates of APC/Fire™ 750, APC/Cyanine7, APC-H7, or APC-eFluor® 780 at each manufacturer's recommended optimal dilution, followed by RBC lysis and wash steps. Histograms were gated on lymphocyte populations based on forward and side scatter.
Low Background Binding to Monocytes
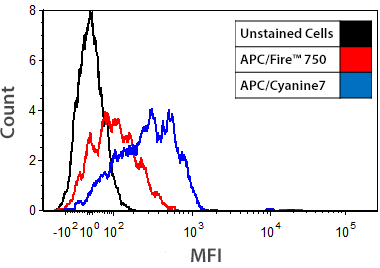
Human whole blood was stained with anti-CD3 conjugates of APC/Fire™ 750 or APC/Cyanine7. Histograms were gated on monocyte populations.
APC/Fire™ 750 Stability
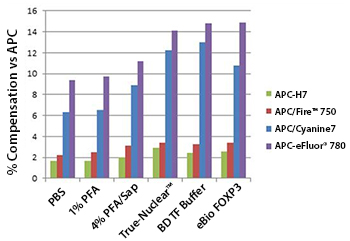 Human whole blood was stained for 20 min with anti-CD3 conjugates of APC/Fire™ 750, APC/Cyanine7, APC-H7, or APC-eFluor® 780 at each manufacturer's recommended optimal dilution, followed by RBC lysis and wash steps. Cells were then treated with either PBS control, 1% paraformaldehyde (PFA) in PBS, 4% PFA followed by 0.1% saponin, BioLegend's True-Nuclear™ Fix/Perm Buffer Set, BD's Transcription Factor Buffer Set, or eBioscience's FOXP3/Transcription Factor Staining Buffer Set prior to analysis. Lymphocyte populations were gated on based on optimal forward and side scatter.
Human whole blood was stained for 20 min with anti-CD3 conjugates of APC/Fire™ 750, APC/Cyanine7, APC-H7, or APC-eFluor® 780 at each manufacturer's recommended optimal dilution, followed by RBC lysis and wash steps. Cells were then treated with either PBS control, 1% paraformaldehyde (PFA) in PBS, 4% PFA followed by 0.1% saponin, BioLegend's True-Nuclear™ Fix/Perm Buffer Set, BD's Transcription Factor Buffer Set, or eBioscience's FOXP3/Transcription Factor Staining Buffer Set prior to analysis. Lymphocyte populations were gated on based on optimal forward and side scatter.
Heat Stability
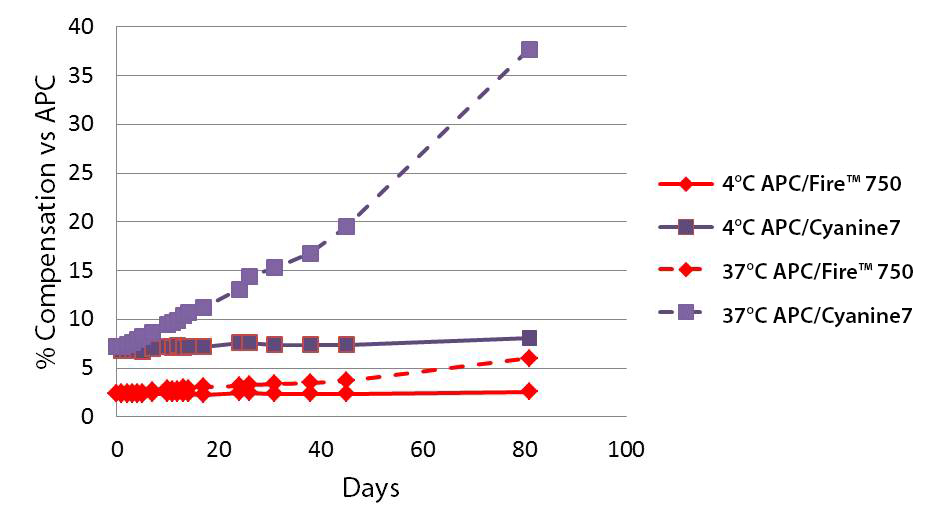 A vial of APC/Cyanine7 or APC/Fire™ 750 was stored in the dark at either 4°C or 37°C for 81 days. At certain intervals, an aliquot of reagent was used to stain Veri-Cells™, a lyophilized human PBMC product that effectively removes donor dependent variation in staining. Cells were stained for 15 min at room temp in cell staining buffer.
A vial of APC/Cyanine7 or APC/Fire™ 750 was stored in the dark at either 4°C or 37°C for 81 days. At certain intervals, an aliquot of reagent was used to stain Veri-Cells™, a lyophilized human PBMC product that effectively removes donor dependent variation in staining. Cells were stained for 15 min at room temp in cell staining buffer.
APC/Fire™ 810
APC/Fire™ 810 expands the range of our spectral detection farther into the infrared than any of our previous fluorophores. On a 5-laser Cytek™ Aurora (UV/V/B/YG/R), APC/Fire™ 810 has very limited spectral spillover into any other channel except PE/Fire™ 810. This means that it can be assigned to any antigen with moderate to abundant expression, and can be used in combination with any other fluorophore. As such, it is very useful for identifying antigens expressed broadly in the cell sample. APC/Fire™ 810 is also very useful for detecting variably expressed markers, like markers of activation, since it emits so far outside of the autofluorescence range. These characteristics make APC/Fire™ 810 potentially very convenient to add into pre-existing panel designs with minimal adverse impact.
Excitation and Emission Spectra of APC/Fire™ 810
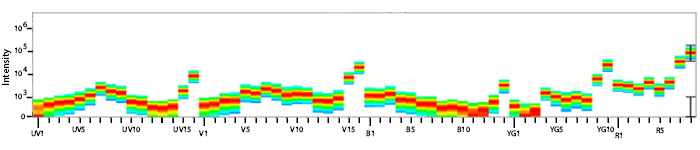
Emission spectra (top) and normalized emission spectra (middle) of APC/Fire™ 810 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare APC/Fire™ 810 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
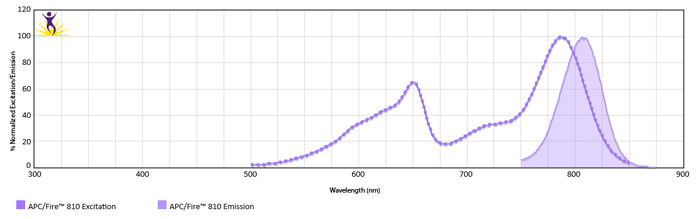
Normalized excitation and emission spectra (bottom) of APC/Fire™ 810 obtained from a spectrophotometer. To compare APC/Fire™ 810 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Spectral Spillover of APC/Fire™ 810
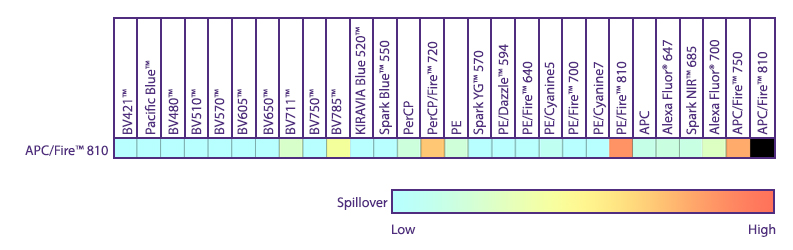
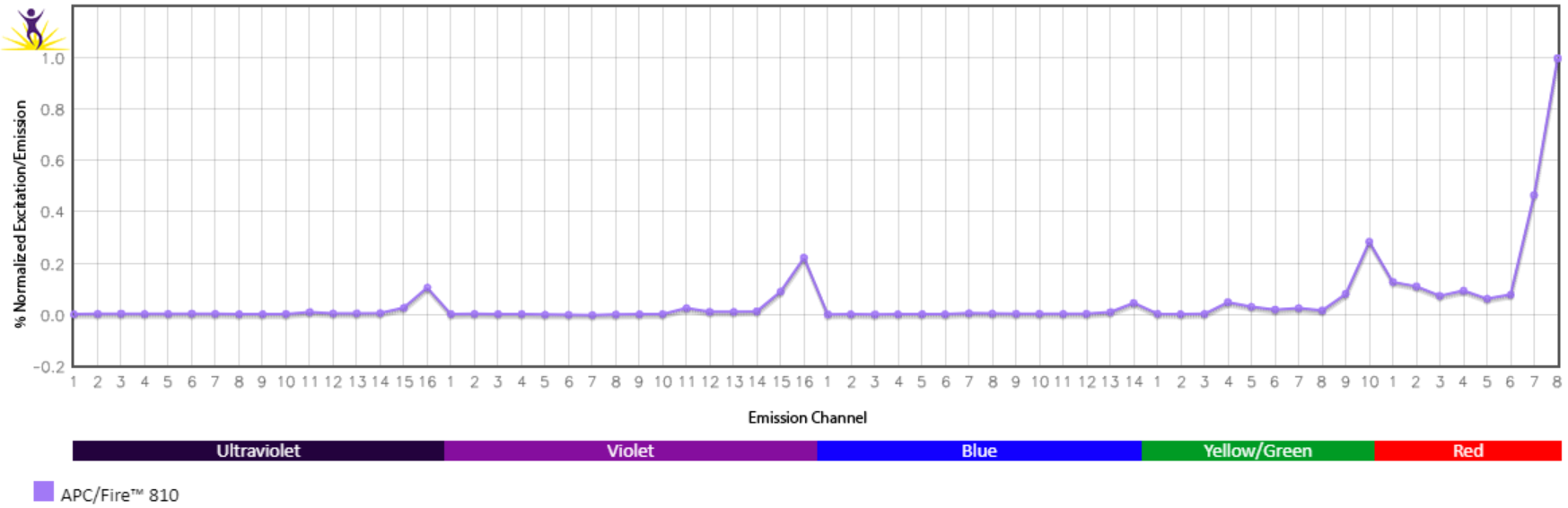
Spillover impact of APC/Fire™ 810 into detection channels of a 5-laser Cytek® Aurora Spectral Cytometer.
Multicolor Compatibility of APC/Fire™ 810
APC/Fire™ 810 Unmixing
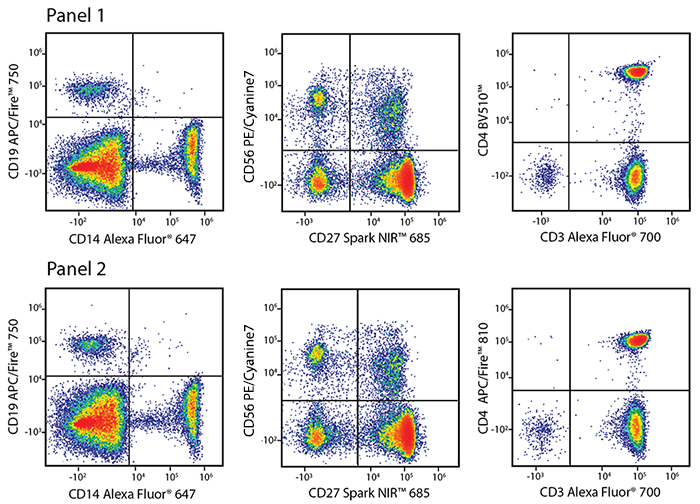
Panel 1:
|
Marker |
Fluorophore |
|---|---|
|
CD4 |
BV510™ |
|
CD3 |
Alexa Fluor® 700 |
|
CD14 |
Alexa Fluor® 647 |
|
CD19 |
APC/Fire™ 750 |
|
CD27 |
Spark NIR™ 685 |
|
CD56 |
PE/Cyanine7 |
Panel 2:
|
Marker |
Fluorophore |
|---|---|
|
CD4 |
APC/Fire™ 810 |
|
CD3 |
Alexa Fluor® 700 |
|
CD14 |
Alexa Fluor® 647 |
|
CD19 |
APC/Fire™ 750 |
|
CD27 |
Spark NIR™ 685 |
|
CD56 |
PE/Cyanine7 |
A common way to test the ability of a fluorophore to be unmixed from other fluorophores with heavily overlapping emission spectra is to create parallel staining panels where the fluorophore of interest (in this case, anti-CD4 APC/Fire™ 810) is replaced with a control antibody conjugated to a fluorophore with non-overlapping spectra (in this case, anti-CD4 BV510™). The accuracy of the unmixing controls, the pattern of the sample staining, and the percent positive populations are all indicators that APC/Fire™ 810 can be cleanly unmixed from all of the other fluorophores currently available for the red laser in this combination. Above are flow plots of RBC-lysed whole human blood cells stained with the two panels.
Intracellular Staining
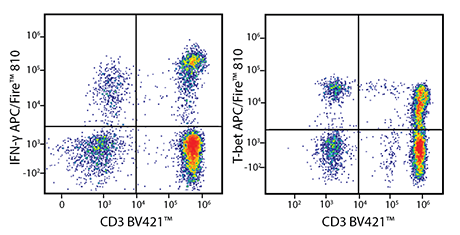
PMA/Ionomycin-stimulated human PBMC were stained with either anti-CD3 BV421™ and anti-IFN-γ APC/Fire™ 810 (left), or with anti-CD3 BV421™ and anti-T-bet APC/Fire™ 810 (right). APC/Fire™ 810 is useful for both intracellular and intranuclear targets. Be aware that when constructing a multicolor panel, APC/Fire™ 810 might exhibit some sensitivity to solvents or oxidation conditions like photobleaching.
Titration Curve for APC/Fire™ 810
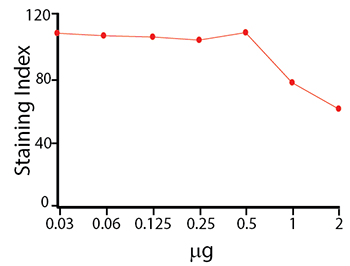
Titration curve of anti-CD4 APC/Fire™ 810 staining on human PBMC.
APC/Fire™ 810 Stability
Photostability
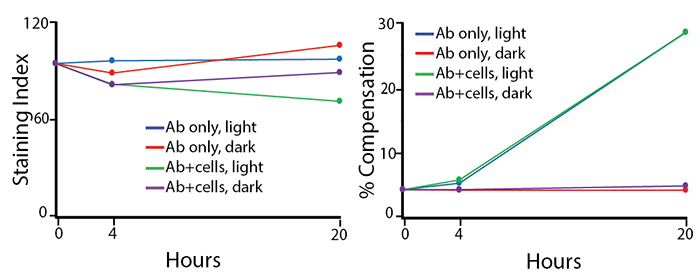
Two conditions of anti-CD4 APC/Fire™ 810 photostability were tested: (1) antibodies formulated at the optimal test concentration were left under fluorescent lighting or protected from light, then used to stain human PBMCs (indicated as “Ab only” in the graphs) and (2) antibodies were used to stain human PBMCs, which were then fixed and left in FluoroFix™ either under fluorescent lighting or protected from light (indicated as “Ab+cells” in the graphs). The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right).
Stability in Fixatives
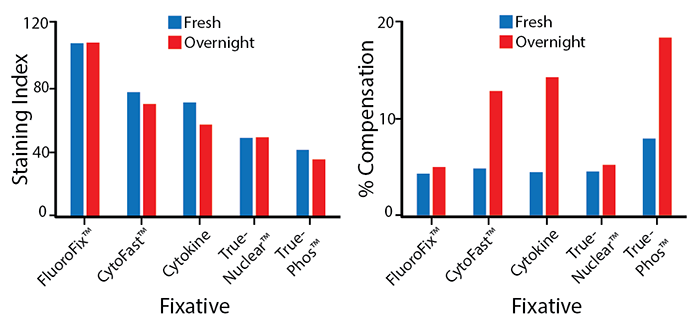
RBC-lysed human blood cells were stained with anti-CD4 APC/Fire™ 810 and fixed according to the recommended protocols for each fixative. Cells were analyzed directly after fixation and washing (fresh), or analyzed after being stored overnight in Cyto-Last™ Buffer. The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right). Learn more about our flow cytometry buffers.
Heat Stability
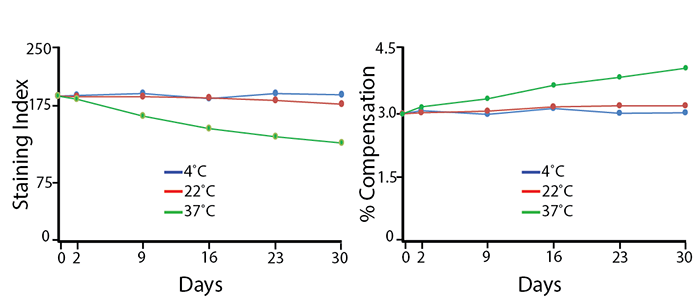
Antibodies were aliquoted into a sealed vial to avoid evaporation and stored at 4°C, 22°C, or 37°C for up to 30 days. The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right). APC/Fire™ 810 has little reduction in staining index over 30 days at room temperature (22°C) and maintains 67% of the original staining index at 37°C after 30 days. The loss of staining index is due to an increase in non-specific binding of the negative population, not a loss of fluorescence intensity. There is a small change in compensation due to loss of acceptor fluors in the tandem.
Note: Percent compensation for each experiment was obtained on a 5L Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
PE/Fire™ 640
Spectral cytometry allows for the simultaneous use of fluorophores with spectral characteristics too similar to be distinguished from each other on conventional cytometers. PE/Fire™ 640 has a distinct emission peak between the peaks of PE/Dazzle™ 594 and PE/Cyanine5, which allows it to be accurately unmixed from these highly overlapping fluorophores. PE/Fire™ 640 was designed to be used for spectral cytometry, but may be used on conventional cytometers with filters optimized by the end user. PE/Fire™ 640 is recommended for: (1) labeling an antigen that is expressed on a small subset of cells in the sample, (2) labeling an antigen that will be excluded from final analysis, and (3) labeling an antigen that requires a bright signal but is not co-expressed with an antigen detected by PE/Cyanine5.
Excitation and Emission Spectra of PE/Fire™ 640
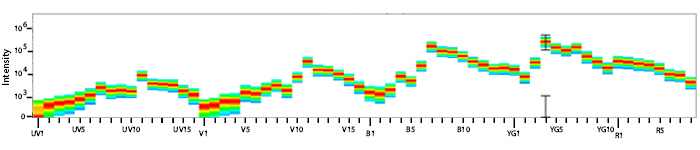
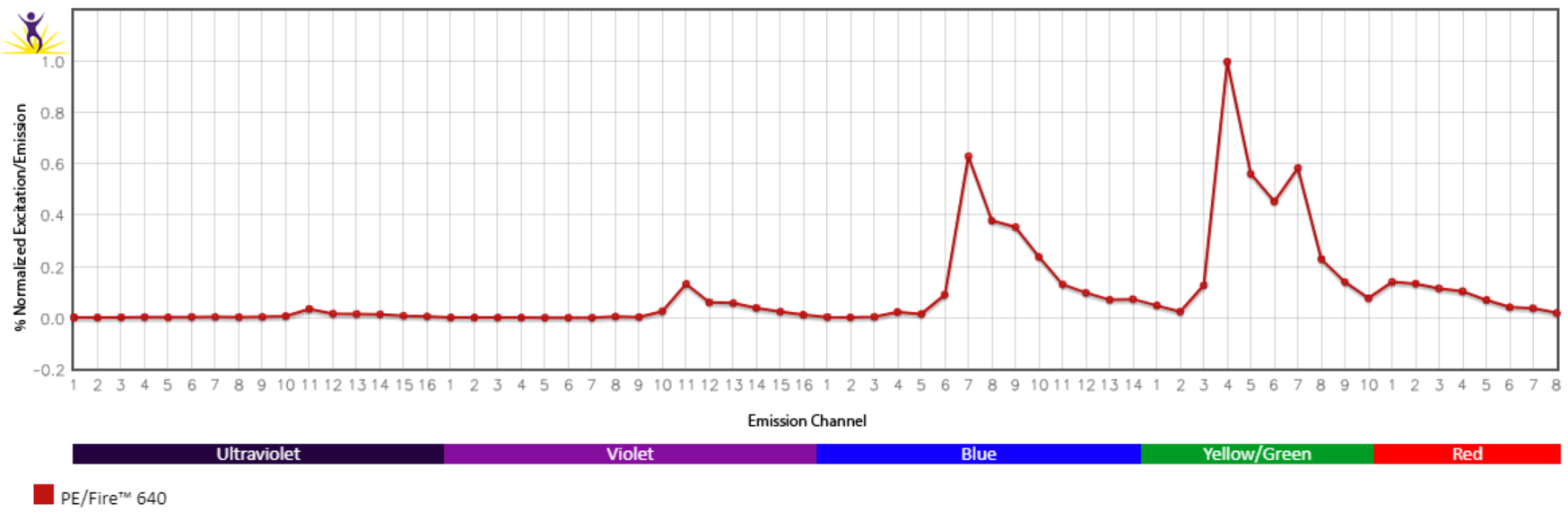
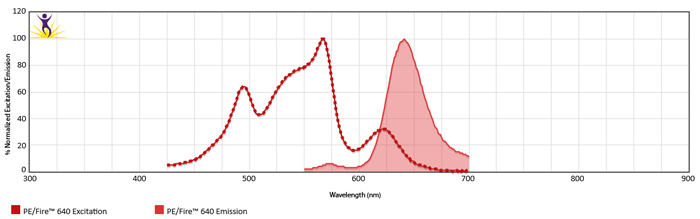
Emission spectra (top) and normalized emission spectra (middle) of PE/Fire™ 640 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PE/Fire™ 640 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PE/Fire™ 640 obtained from a spectrophotometer. To compare PE/Fire™ 640 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Spectral Spillover of PE/Fire™ 640
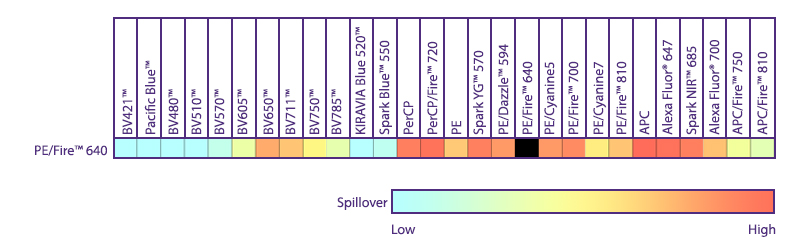
Spillover impact of PE/Fire™ 640 into detection channels of a 5-laser Cytek® Aurora Spectral Cytometer.
Multicolor Compatibility of PE/Fire™ 640
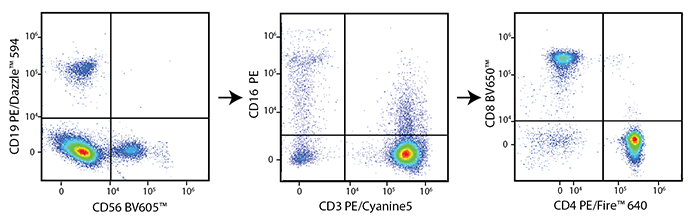
Multicolor Panel:
|
Marker |
Clone |
Fluorophore |
|---|---|---|
|
CD4 |
SK3 |
PE/Fire™ 640 |
|
CD3 |
UCHT1 |
PE/Cyanine5 |
|
CD19 |
HIB19 |
PE/Dazzle™ 594 |
|
CD56 |
HCD56 |
BV605™ |
|
CD16 |
3G8 |
PE |
|
CD8 |
RPA-T8 |
BV650™ |
RBC-lysed and washed human blood was stained with optimal test concentrations of the antibodies listed in the left panel. Blocking buffers were used as appropriate.
Titration Curve for PE/Fire™ 640
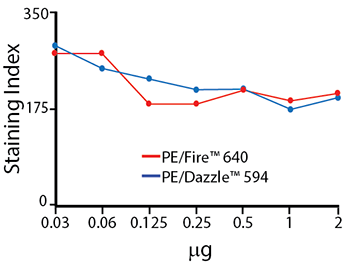
Titration curves for anti-CD4 PE/Dazzle™ 594 and anti-CD4 PE/Fire™ 640. Antibodies were titrated from 0-2 μg/test, and used to stain RBC-lysed human blood. Cells were analyzed on a Cytek® Aurora.
PE/Fire™ 640 Stability
Photostability
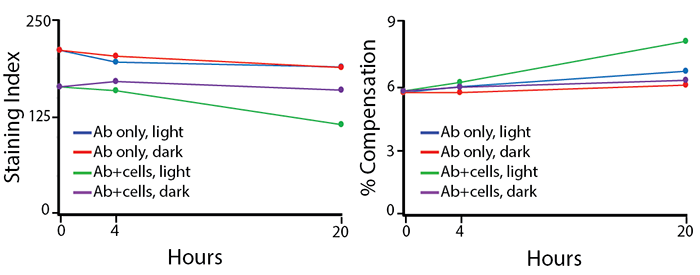
Two conditions of anti-CD4 PE/Fire™ 640 photostability were tested: (1) antibodies formulated at the optimal test concentration were left under fluorescent lighting or protected from light, then used to stain human PBMCs (indicated as “Ab only” in the graphs) and (2) antibodies were used to stain human PBMCs, which were then fixed and left in FluoroFix™ either under fluorescent lighting or protected from light (indicated as “Ab+cells” in the graphs). The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right).
Stability in Fixatives
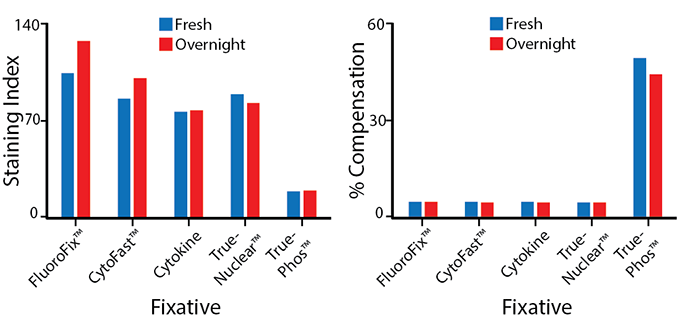
RBC-lysed human blood cells were stained with anti-CD4 PE/Fire™ 640 and fixed according to the recommended protocols for each fixative. Cells were analyzed directly after fixation and washing (fresh), or analyzed after being stored overnight in Cyto-Last™ Buffer. PE/Fire™ 640 is resistant to paraformaldehyde fixation, but is sensitive to organic solvent-containing phospho-specific fix and perm buffers (e.g. True-Phos™). The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right). Learn more about our flow cytometry buffers.
Heat Stability
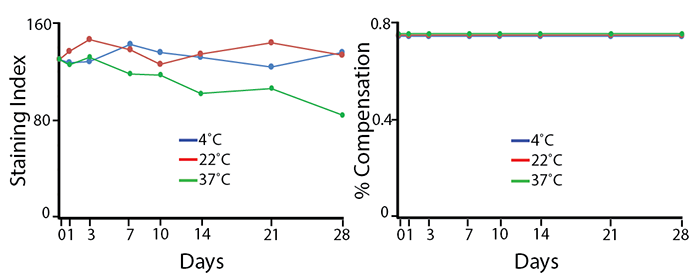
Antibodies were aliquoted into a sealed vial to avoid evaporation and stored at 4°C, 22°C, or 37°C for up to 28 days. The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right). PE/Fire™ 640 does not have a significant reduction in staining index over 28 days at room temperature (22°C), but is reduced by 40% in staining index after 28 days at 37°C. There is no change in compensation, and the loss of staining index is due to an increase in non-specific binding of the negative population, not a loss of fluorescence intensity.
Note: Percent compensation for PE/Fire™ 640 heat stability was obtained on a conventional flow cytometer. Percent compensation for each other experiment was obtained on a 5L Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
PE/Fire™ 700
PE/Fire™ 700 ranks high on the brightness scale, comparable to PE/Cyanine5.5. Due to its significant spillover, PE/Fire™ 700 may be best suited for detecting antigens that are lowly and discreetly expressed. For example, the brightness of PE/Fire™ 700 can be leveraged to identify markers of rare populations that are not expressed on more abundant cell types. For both spectral and conventional cytometry, PE/Fire™ 700 can serve as a more stable alternative to PE/Cyanine5.5.
Excitation and Emission Spectra of PE/Fire™ 700
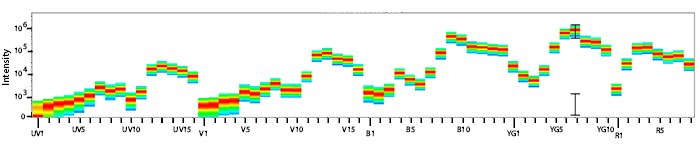
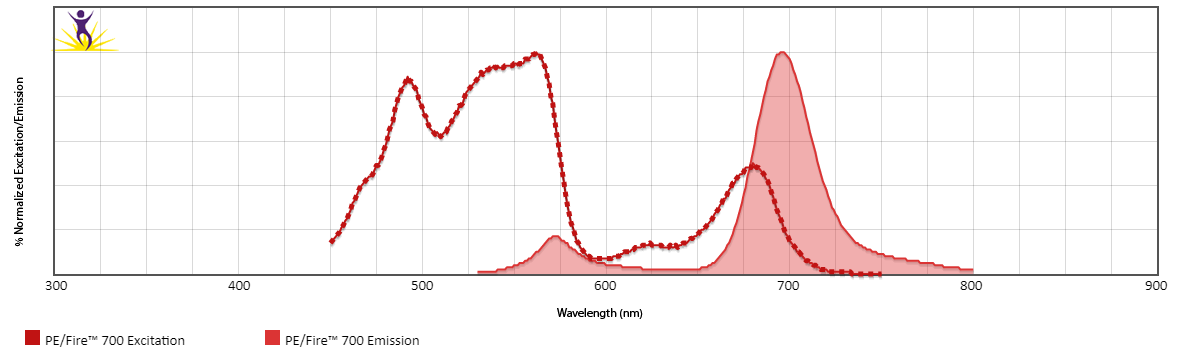
Emission spectra (top) and normalized emission spectra (middle) of PE/Fire™ 700 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PE/Fire™ 700 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PE/Fire™ 700 obtained from a spectrophotometer. To compare PE/Fire™ 700 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
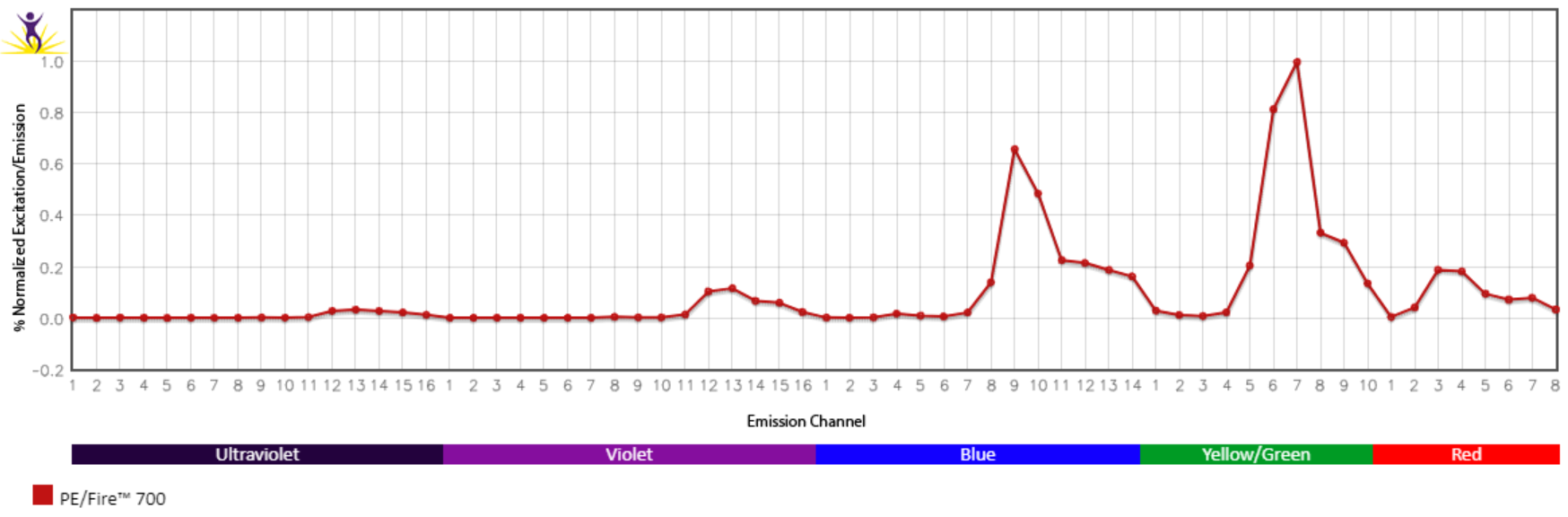
Spectral Spillover of PE/Fire™ 700
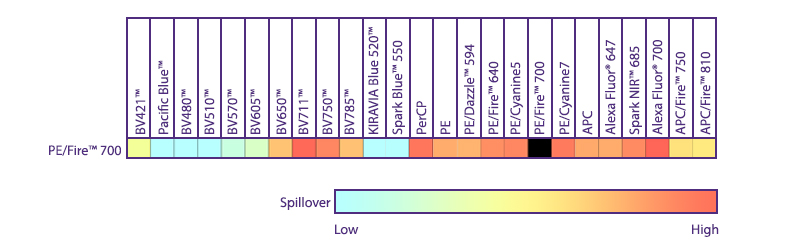
Spillover impact of PE/Fire™ 700 into detection channels of a 5-laser Cytek® Aurora Spectral Cytometer.
Multicolor Compatibility of PE/Fire™ 700
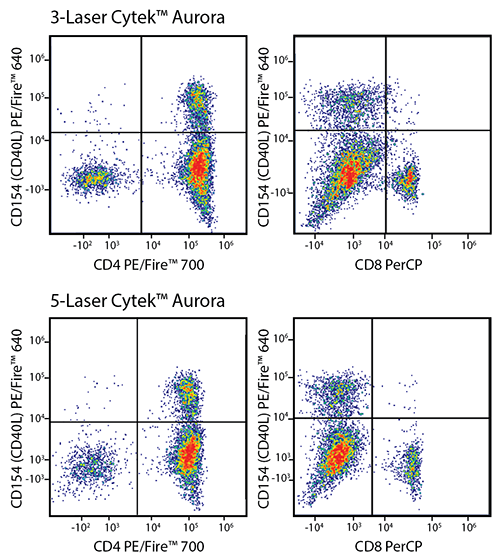
Multicolor Panel:
|
Marker |
Clone |
Fluorophore |
|---|---|---|
|
CD4 |
SK3 |
PE/Fire™ 700 |
|
CD8 |
SK1 |
PerCP |
|
CD19 |
HIB19 |
PE/Cyanine5 |
|
CD56 |
5.1H11 |
PE/Dazzle™ 594 |
|
CD154 |
24-31 |
PE/Fire™ 640 |
RBC-lysed and washed human blood was stained with a multicolor panel with an intentionally high degree of spectral complexity and overlap (left). Cells were analyzed on a 3-laser (top panels) or 5-laser Cytek® Aurora (bottom panels). Optimal test size concentrations were used for each antibody. Cells were gated on CD19-/CD56- events, and CD154 (CD40L) PE/Fire™ 640 is displayed against CD4 PE/Fire™ 700 or CD8 PerCP. This panel is able to be unmixed on both a 3-laser and a 5-laser Aurora. However, both the intensity of the PerCP signal and the spreading error of PE/Fire™ 640 is less substantial using a system that has both a 488 nm blue laser and a 561 nm yellow/green laser (Bottom panels). Spreading error only poses a problem when the brightness of the two fluors being plotted is not of sufficient intensity to resolve the two populations.
PE/Fire™ 700 Unmixing from PE/Cyanine5.5
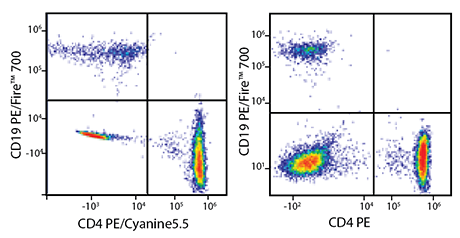
RBC-lysed and washed human blood was co-stained with anti-CD19 PE/Fire™ 700 and either anti-CD4 PE/Cyanine5.5 (left) or a non-overlapping control fluor – in this case, anti-CD4 PE (right). Cells were acquired on a 5-laser Cytek® Aurora and gated on lymphocytes. When compared to the non-overlapping control (right), cells co-stained with PE/Fire™ 700 and PE/Cyanine5.5 (left) display a similar pattern of percent positive populations. This shows that it is possible to unmix PE/Fire™ 700 from PE/Cyanine5.5, but panel design should be carefully considered.
PE/Fire™ 700 and PE/Cyanine5.5 Brightness Comparison
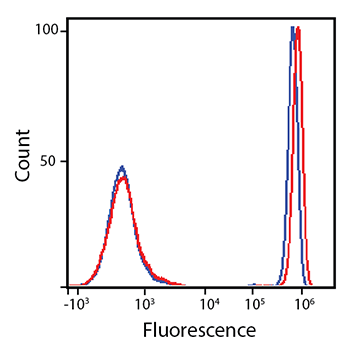
RBC-lysed and washed human blood was stained with anti-CD4 PE/Fire™ 700 (red) or anti-CD4 PE/Cyanine5.5 (blue).
Titration Curve for PE/Fire™ 700
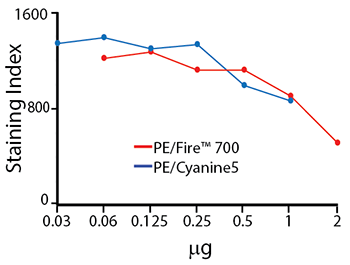
Titration of anti-CD4 PE/Fire™ 700 and anti-CD4 PE/Cyanine5 staining on RBC-lysed and washed human blood. Titrations were performed within the optimal range for each individual fluorophore. These two fluorophores are similar in brightness but both also exhibit significant spillover into neighboring emission channels. The staining index tends to decrease with greater concentrations of antibody used – this is due to an increase in non-specific binding.
PE/Fire™ 700 Stability
Photostability
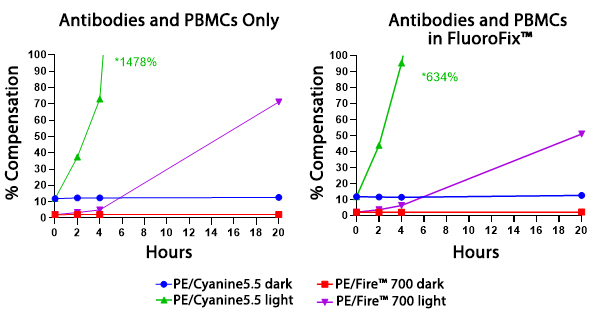
Two conditions of anti-CD4 PE/Fire™ 700 and anti-CD4 PE/Cyanine5.5 photostability were tested: (left) antibodies formulated at the optimal test concentration were left under fluorescent lighting or protected from light, then used to stain human PBMCs and (right) antibodies were used to stain human PBMCs, which were then fixed and stored in FluoroFix™ either under fluorescent lighting or protected from light. The long-term stability of antibody conjugates can be predicted by its brightness and, in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel.
Stability in Fixatives
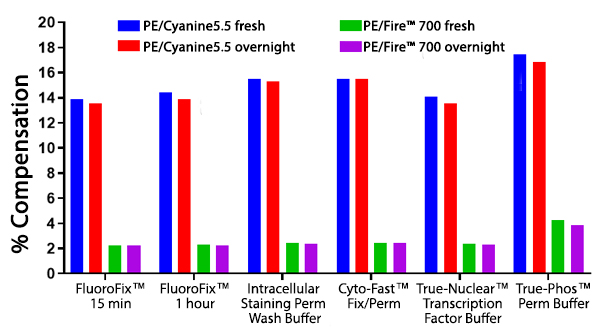
RBC-lysed and washed human blood cells were stained with anti-CD4 PE/Fire™ 700 or anti-CD4 PE/Cyanine5.5 and fixed according to the recommended protocols for each fixative. Cells were analyzed directly after fixation and washing (fresh), or analyzed after being stored overnight in Cyto-Last™ Buffer. PE/Fire™ 700 is resistant to paraformaldehyde fixation but is sensitive to organic solvent-containing phospho-specific fix and perm buffers (e.g. True-Phos™). The long-term stability of antibody conjugates can be predicted by its brightness, and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel. Learn more about our flow cytometry flow cytometry buffers.
Heat Stability
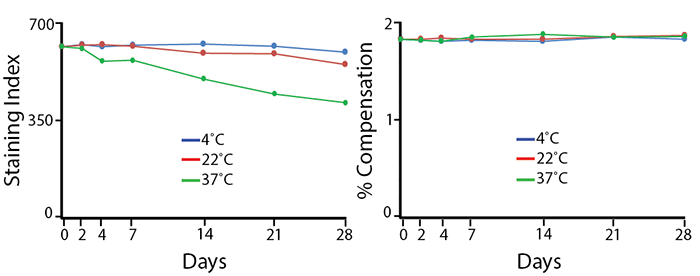
Antibodies were aliquoted into a sealed vial to avoid evaporation and stored at 4°C, 22°C, or 37°C for up to 28 days. The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right). PE/Fire™ 700 exhibits a reduction of 12% in staining index over 28 days at room temperature (22°C) and 36% reduction in staining index at 37°C. There is no change in compensation and the loss of staining index is due to an increase in non-specific binding of the negative population, not a loss of fluorescence intensity.
Note: Percent compensation for each experiment was obtained on a 5-laser Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
PE/Fire™ 744
PE/Fire™ 744 is an extremely bright tandem dye that is excited by both the blue and yellow/green lasers. On average, it is brighter than PE/Cyanine7, but exhibits less spillover back into the PE donor channel. PE/Fire™ 744 can be used as an alternative to PE/Cyanine7 on conventional instruments and can be unmixed from PerCP/Fire™ 780 and PerCP/Fire™ 806 on spectral instruments. Due to its unique spectral signature, it can even be unmixed from PE/Cyanine7 with careful panel design and analysis. PE/Fire™ 744 may exhibit non-specific binding to monocytes. True-Stain Monocyte Blocker™ (Cat No. 426102) is recommended to minimize non-specific staining.
Excitation and Emission Spectra of PE/Fire™ 744
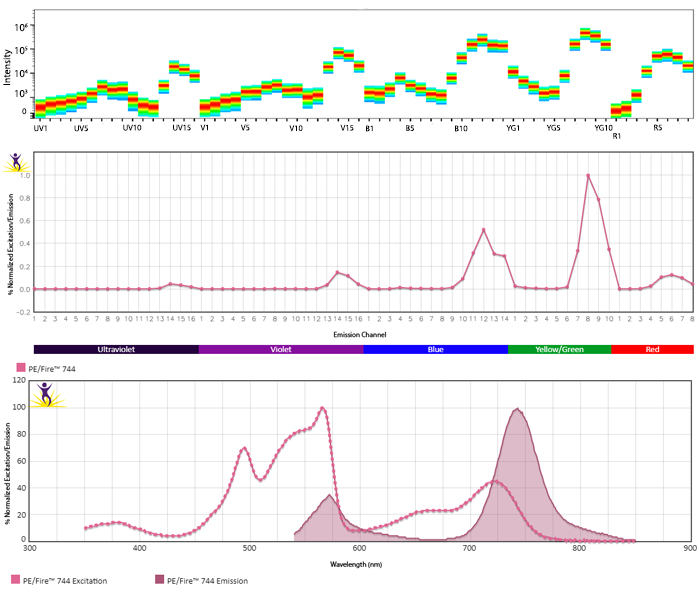
Emission spectra (top) and normalized emission spectra (middle) of PE/Fire™ 744 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PE/Fire™ 744 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PE/Fire™ 744 obtained from a spectrophotometer. To compare PE/Fire™ 744 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Multicolor Compatibility of PE/Fire™ 744
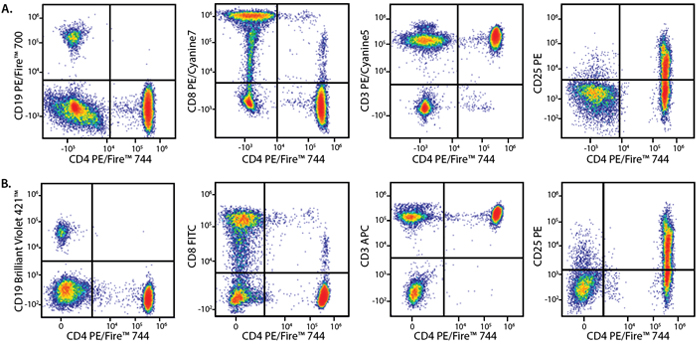
Panel A:
|
Marker |
Fluorophore |
|---|---|
|
CD3 |
PE/Cyanine5 |
|
CD4 |
PE/Fire™ 744 |
|
CD8 |
PE/Cyanine7 |
|
CD19 |
PE/Fire™ 700 |
|
CD25 |
PE |
Panel B:
|
Marker |
Fluorophore |
|---|---|
|
CD3 |
APC |
|
CD4 |
PE/Fire™ 744 |
|
CD8 |
FITC |
|
CD19 |
BV421™ |
|
CD25 |
PE |
Human lysed whole blood was stained with a multicolor panel with either a high (Panel A) or low (Panel B) degree of spectral complexity and overlap. Samples were analyzed on a 5-laser Cytek® Aurora cytometer. All plots are gated on lymphocytes.
PE/Fire™ 744 Stability
Photostability
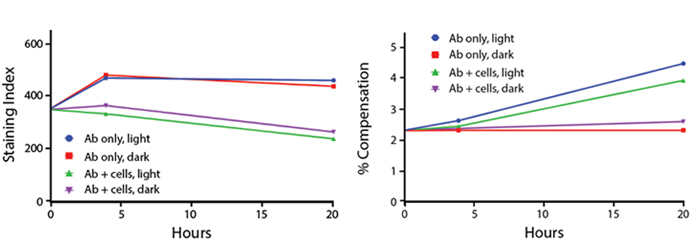
Anti-human CD4 (clone SK3) PE/Fire™ 744 was stored in clear vials (Ab only) and left exposed to light or protected in the dark, as indicated. Antibodies were stored for the indicated time points and then used to stain human lysed whole blood. Samples labeled "Ab + cells" contain human lysed whole blood stained with anti-human CD4 PE/Fire™ 744. Stained cells were then left in the light or protected, as indicated.
Stability in Fixatives
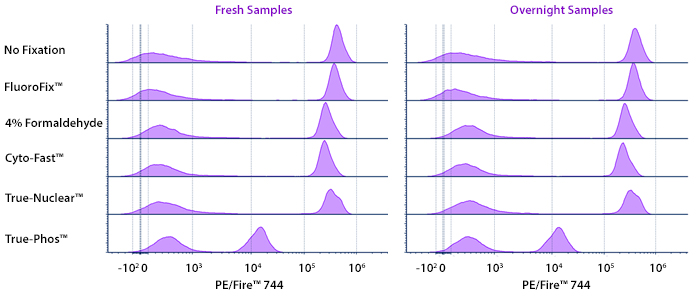
Human PBMCs were stained with anti-human CD4 (clone SK3) conjugated to PE/Fire™ 744 and fixed using the respective protocols for each buffer set. Fresh samples were fixed and read on a 5-laser Cytek® Aurora Cytometer immediately. Overnight samples were fixed and stored overnight in Cyto-Last™ Buffer before reading.
Heat Stability
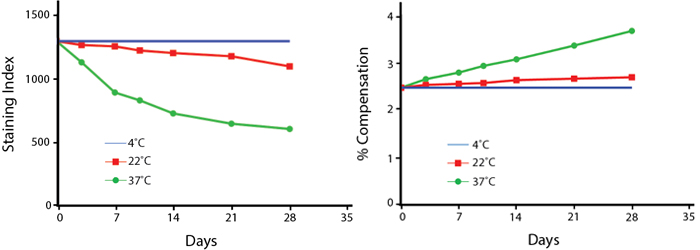
Anti-human CD4 (clone SK3) PE/Fire™ 744 was aliquoted and incubated at the indicated temperatures over the course of 28 days. The antibodies were then used to stain human lysed whole blood from a single donor.
Note: Percent compensation for each experiment was obtained on a 5-laser Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
PE/Fire™ 810
Along with APC/Fire 810™, PE/Fire™ 810 expands the range of spectral detection further than any of our previous fluorophores, emitting even beyond the range of PE/Cyanine7. Optimized for spectral unmixing cytometers, PE/Fire™ 810 is excited by the blue and yellow/green lasers and can utilized for nearly any antigen since it has little spectral overlap with other fluorophores. While we have released several PE tandem options, our data below shows PE/Fire™ 810 can be unmixed from them in a multicolor panel.
Excitation and Emission Spectra of PE/Fire™ 810
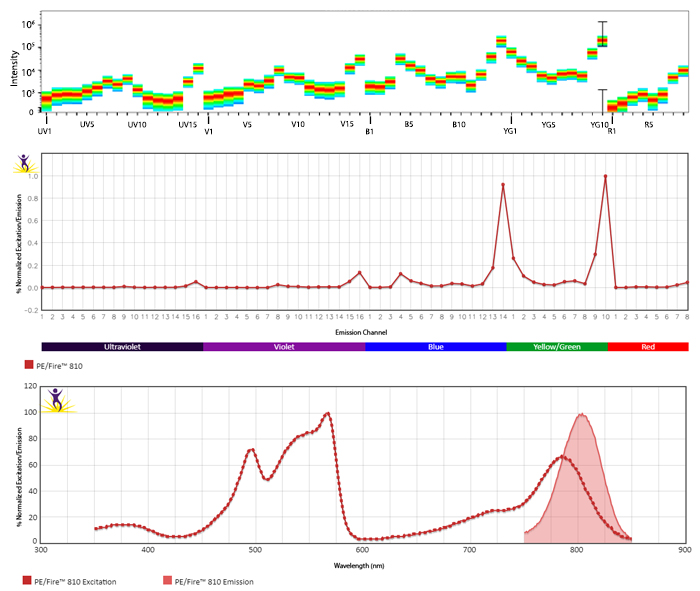
Emission spectra (top) and normalized emission spectra (middle) of PE/Fire™ 810 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PE/Fire™ 810 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PE/Fire™ 810 obtained from a spectrophotometer. To compare PE/Fire™ 810 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Multicolor Compatibility of PE/Fire™ 810
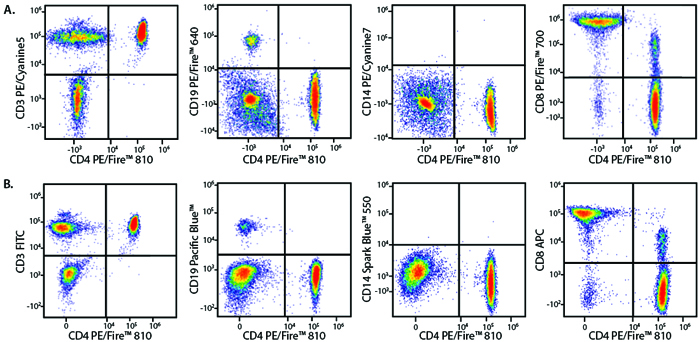
Panel A:
|
Marker |
Fluorophore |
|---|---|
|
CD3 |
PE/Cyanine5 |
|
CD4 |
PE/Fire™ 810 |
|
CD8 |
PE/Fire™ 700 |
|
CD14 |
PE/Cyanine7 |
|
CD19 |
PE/Fire™ 640 |
Panel B:
|
Marker |
Fluorophore |
|---|---|
|
CD3 |
FITC |
|
CD4 |
PE/Fire™ 810 |
|
CD8 |
APC |
|
CD14 |
Spark Blue™ 550 |
|
CD19 |
Pacific Blue™ |
RBC-lysed and washed human blood lymphocytes were stained with a multicolor panel with either a high (Panel A) or low (Panel B) degree of spectral complexity and overlap. CD4 vs. CD8 plots are also gated on the CD3+ lymphocyte population.
PE/Fire™ 810 Stability
Photostability
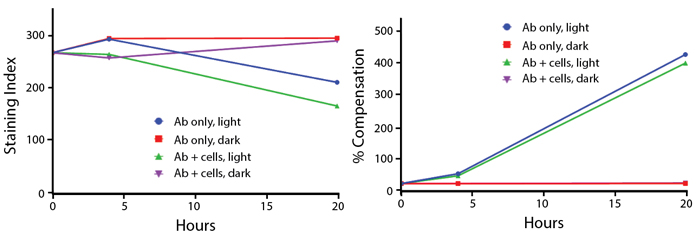
Anti-human CD4 (clone SK3) PE/Fire™ 810 was stored in a clear vial (Ab only) and left exposed to light or protected in the dark, as indicated. Antibodies were stored for the indicated time points and then used to stain human lysed whole blood. Samples labeled "Ab + cells" contain human lysed whole blood that was stained with anti-human CD4 PE/Fire™ 810. Stained cells were then left in the light or protected, as indicated. Note that for the % Compensation graph, the Ab only, dark (red) and Ab + cells, dark (purple) data points were very similar, causing the red line to overlap with the purple line.
Stability in Fixatives
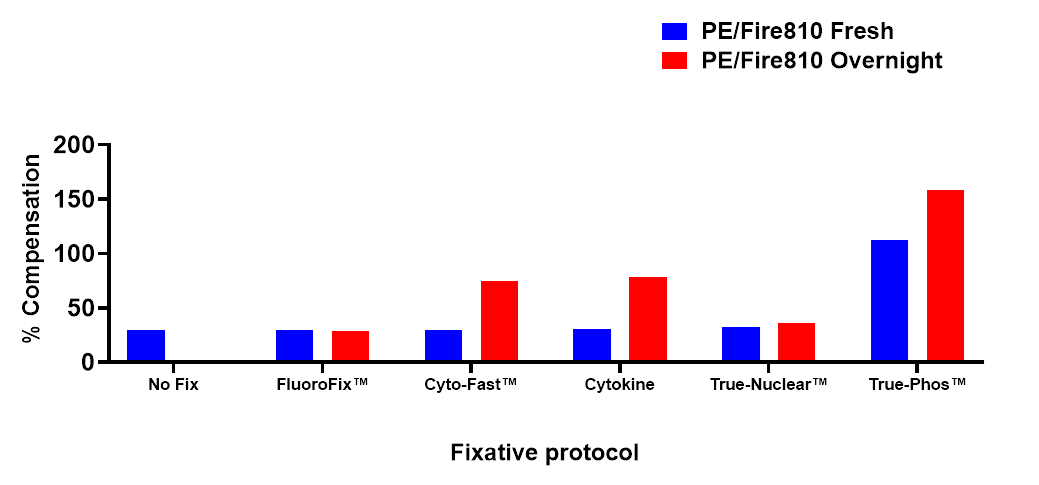
Human PBMCs were stained with anti-human CD4 (clone SK3) conjugated to PE/Fire™ 810 and fixed using the respective protocols for each buffer set. Fresh samples were fixed and read on a 5-laser Cytek™ Aurora Cytometer immediately. Overnight samples were fixed and stored overnight in FluoroFix™ Buffer before reading.
Heat Stability
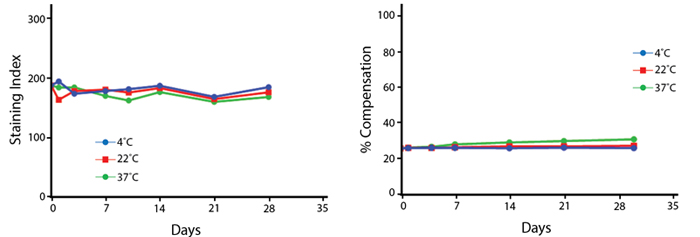
Anti-human CD4 (clone SK3) PE/Fire™ 810 was aliquoted and incubated at the indicated temperatures over the course of 30 days. The antibodies were then used to stain human lysed whole blood from a single donor.
Note: Percent compensation for each experiment was obtained on a 5-laser Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
 Login / Register
Login / Register 






Follow Us