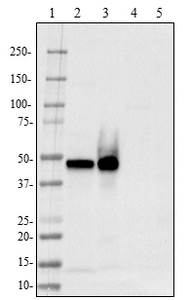
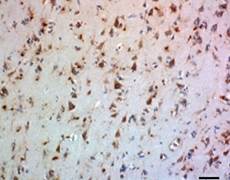
Alzheimer's disease is characterized by the development of amyloid beta (Aβ) plaques, which can cause neurotoxicity. These plaques are made of Aβ fibrils and are generated by the improper cleavage of Amyloid Precursor Protein. Aβ fibrils can join with other proteins to create β-sheets resistant to proteolysis. However, these plaques are not invulnerable to dissociation. Microglia in the brain can take up amyloid fibrils by endocytosis. As noted by Solé-Doménech et al., tripeptidyl peptidase 1 (TPP1) is capable of cleaving β-sheets in fibrils at multiple sites. This exposes the fibril to 60+ hydrolases for further degradation.
BioLegend offers a new highly human-specific monoclonal antibody, clone 2E12, for TPP1 detection in western blotting and immunohistochemistry of paraffin embedded sections. With improved reagents, this enzyme can be studied more efficiently to understand the degradation of Aβ and how to limit the effects of Alzheimer's disease.

Adapted from Solé-Doménech et al. 2018. Proc. Natl. Acad. Sci. USA. 115:1493. Pubmed
|





 Login / Register
Login / Register 








Follow Us