NRF2 Signaling in Inflammation and Disease
The Nuclear factor‐erythroid 2 p45‐related factor 2 (NRF2) protein has been described as a master regulator of redox metabolism due to its key role in the cell’s response to oxidative stress (1,2) and has recently been implicated in many other processes such as proteostasis, neurodegeneration, and inflammation (2,5,7).
Activation of NRF2 has been shown to have a beneficial role in various disorders, making it an attractive candidate for therapeutic research and clinical development (5-8).
BioLegend offers reagents for studying NRF2 in multiple applications and research areas. Contact us to learn more.
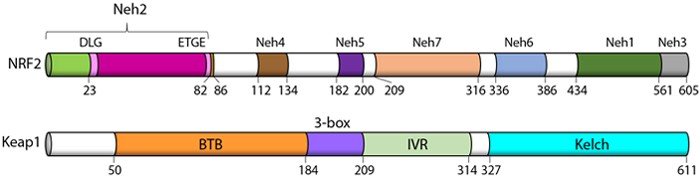
Adapted from, Canning, P., et al. 2015. Free Radical Biology and Medicine. Vol 88. B:101.
Transcription factors regulate gene expression by binding to DNA and causing stimulation or repression of transcription. NRF2 target genes encode a variety of antioxidant enzymes and proteins involved in detoxification, repair and removal of damaged proteins and organelles, inflammation, and mitochondrial bioenergetics. NRF2 also controls the expression of key components of the glutathione (GSH) and thioredoxin (TXN) antioxidant system, enzymes involved in NADPH regeneration, and reactive oxygen species (ROS). NRF2 also helps regulate xenobiotic detoxification and heme metabolism, playing a fundamental role in maintaining the redox homeostasis of the cell (2,7).
Under normal conditions, NRF2 levels are kept low due to ubiquitination and degradation by Cul3, the E3 ubiquitin ligase Keap1, and the proteasome.
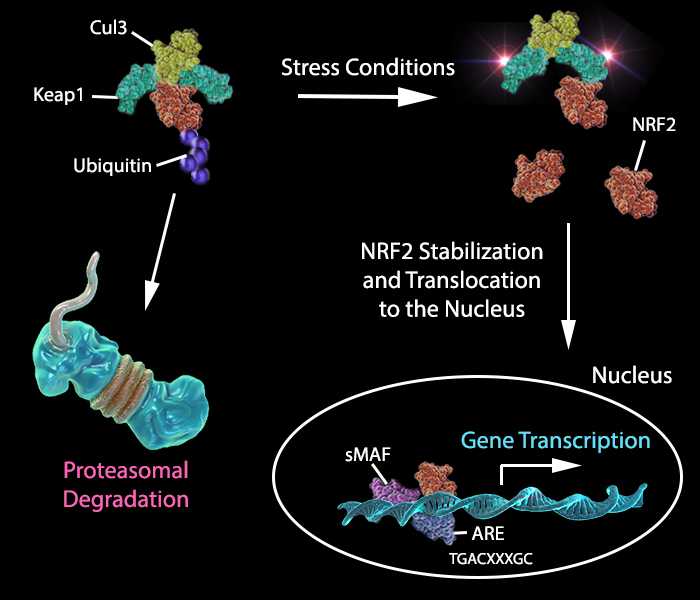
Adapted from Tonelli, C. et al. 2018. Antioxidants and Redox Signaling. Vol 29:17.
In response to oxidative stress, electrophile metabolites inhibit activity of the Keap1 complex, promoting nuclear accumulation of NRF2 and binding to AREs (antioxidant response elements) of cytoprotective target genes (Figure 2), such as detoxifying enzymes, leading to neutralization of reactive electrophiles (2).
NRF2 is frequently activated in many types of cancers and is thought to drive metabolic adaptation and survival in ROS-rich tumor microenvironments. The NRF2 pathway is also activated in response to selective autophagy, or the removal of misfolded proteins or organelles. Autophagy promotes interaction between Keap1 and SQSTM1/p62 and subsequent inactivation of the Keap1 complex, also leading to NRF2 nuclear accumulation and expression of cytoprotective genes.
In stem cells, NRF2 regulates self-renewal through interaction with important factors such as cyclinD1, OCT4, Nanog, and Pax6 (3). NRF2 inhibition leads to cell differentiation and a loss of renewal, suggesting an important role in stem cell development (3).
The Role of NRF2 in Inflammation
Under stress, activation of NRF2 promotes resolution of inflammation through repression of proinflammatory cytokines (TNF‐α, IL‐1, IL‐6, IL‐8, MCP‐1), restores cellular redox and protein homeostasis, and facilitates tissue repair. This makes it an active area of investigation in inflammation and neurodegenerative disorders.
In SARS-CoV-2-induced infection, the inflammatory response is a crucial determinant of disease outcome and correlates with disease severity. Acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 is thought to be the result of a dysregulated host response, followed by lung damage in alveolar cells and fibrosis. The most aggressive presentations are characterized by exacerbated proinflammatory cytokine release and leukopenia, the loss of T lymphocytes. Activators of NRF2 have been proposed as potential therapies for symptoms of SARS-CoV-2 infection (5).
Our poster, SARS-CoV-2 Biology and Reagents, describes several topics associated with the virus, including viral entry, viral replication methods, and cytokine release syndrome, and highlights the ways our research reagents help scientists understand it. Download or request a copy here.
NRF2 function is altered in a number of neurodegenerative disorders, such as Alzheimer's disease, amyotrophic lateral sclerosis (ALS), Huntington's disease and Friedreich's ataxia (6,7). Activation of NRF2 has been shown to mitigate multiple pathogenic processes involved in these neurodegenerative disorders through upregulation of antioxidant defenses, inhibition of inflammation and improvement of mitochondrial function (6,7,8).
Inflammation in the brain (neuroinflammation) results from acute injury or following the accumulation of mutant proteins or endogenous neurotoxic metabolites such as those associated with neurodegenerative diseases. Microglia, in particular, play a key role in brain inflammation via the release of proinflammatory cytokines. Increased neuroinflammation and oxidative stress following microglial activation are associated with age‐related cognitive impairment (7). Recent evidence suggests a mechanism of transcriptional repression of proinflammatory cytokines in microglia, macrophages, monocytes, and astrocytes following NRF2 activation (6).
Small molecule activators of NRF2 have shown protective effects in various animal models of neurodegeneration, and in cultures of human cells expressing mutant proteins (6,8). Targeting NRF2 signaling may provide a therapeutic option to delay onset, slow progression, and ameliorate symptoms of neurodegenerative disorders (6,7,8).
BioLegend provides solutions for advancing all aspects of your research. Explore our website to learn more about the roles of transcription factors in cell biology or contact our experts to learn about our products.
References:
- Hayes, J.D. and Dinkova-Kostova, A.T. 2014. Trends Biochem Sci. 39:199. The NRF2 regulatory network provides an interface between redox and intermediary metabolism. DOI: 10.1016/j.tibs.2014.02.0023
- Kobayashi E.H. et al. 2016. Nat Commun 7(1):11624. NRF2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. DOI: 10.1038/ncomms11624
- Tonelli C. et al. 2018. Antioxidants & Redox Signaling. 29(17):1727. Transcriptional Regulation of NRF2. DOI: 10.1089/ars.2017.7342
- Dai, X. et al. 2020. Trends in Molecular Medicine. 26(2):185. NRF2: Redox and Metabolic regulator of stem cell state and function. DOI: 10.1016/j.molmed.2019.09.007
- Cuadrado, A. et al. 2020. Trends in Pharmacological Sciences. 41(9):598. Can activation of NRF2 be a strategy against COVID-19? DOI: 10.1016/j.tips.2020.07.003
- Dinkova-Kostova, A.T. et al. 2018. The FEBS journal. 285(19):3576. The role of NRF2 signaling in counteracting neurodegenerative diseases. DOI: 10.1111/febs.14379
- Robledinos-Antón, N. et al. 2019. Oxidative medicine and cellular longevity. Activators and inhibitors of NRF2: a review of their potential for clinical development. DOI: 10.1155/2019/9372182
- Brandes, M. and Gray, N. 2020. Asn Neuro. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. DOI: 10.1177/1759091419899782
- Lo, S. et al. 2006. The EMBO journal. 25.15: 3605-3617. Structure of the Keap1: Nrf2 interface provides mechanistic insight into Nrf2 signaling. DOI:10.1038/sj.emboj.7601243
- Canning, P. et al. 2015. Free radical biology & medicine. 88. B:101. Structural basis of Keap1 interactions with Nrf2. DOI:10.1016/j.freeradbiomed.2015.05.034
 Login / Register
Login / Register 






Follow Us