End-to-End Solutions for T Cell Manufacturing Applications
Our immune system plays a central role in combating cancer. However, tumor cells can avoid immune attack and progress to cancer. Adoptive cell therapies (ACTs) are turning the tide with the help of T and natural killer (NK) cells engineered with chimeric antigen receptors (CARs) to target specific tumors. CAR-T and CAR-NK cells precisely recognize tumor cells and kill them in a targeted manner. Browse our resources to learn more about advanced cancer treatments1 such as CAR-T or CAR-NK cells, monoclonal antibodies, and immune checkpoint inhibitors.
In this blog, we discuss CAR-T cell cancer immunotherapy, a type of ACT, and the comprehensive workflow solutions offered by BioLegend, now part of Revvity, to support cell processing and downstream manufacturing.
CAR-T/NK cell manufacturing is a complex, multi-step process that requires control and consistency throughout to produce batches of T or NK cells with the desired characteristics2 (see illustration below). The process begins with isolating and purifying cells from a patient (autologous) or healthy donor(s) (allogeneic). Isolated cells are then activated, engineered, and expanded in lab and then infused into the patient to treat the cancer.
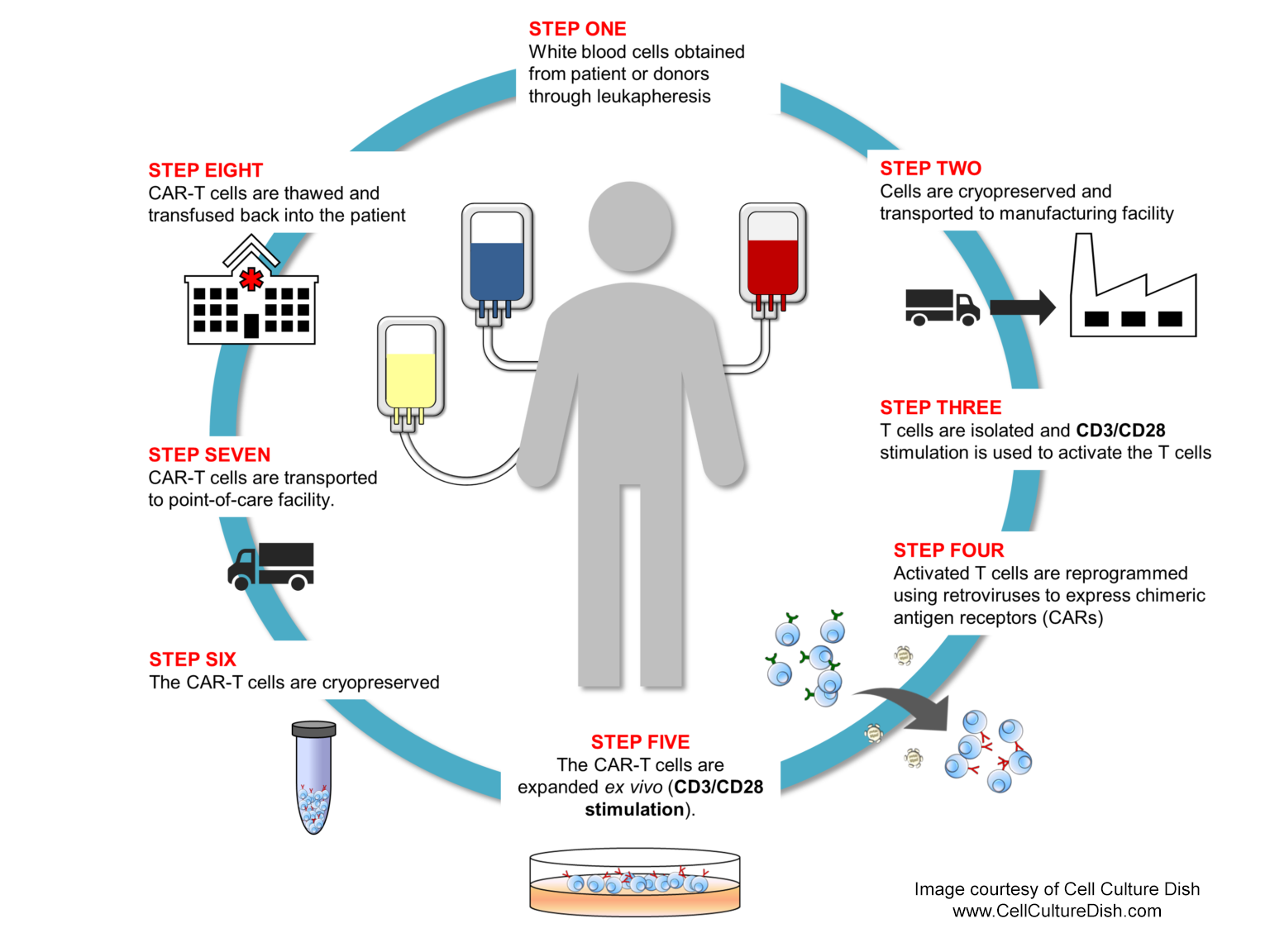
In the next section, we navigate researchers through cell manufacturing steps and highlight the solutions we designed to play an early role in controlling the overall quality, safety, and efficacy of the final cell products.
Check cell viability and quality with our GMP isolation reagents.
Analyze the enriched fraction: get cell counts and determine immune cell composition using TBNK panels to identify T cells, B cells, NK cells, and NKT cells.
preservation
and
engineering
Achieve multiplexed immunogen knockout and CAR knock in with the Pin-point™ base editing platform.
and
Culture
Culture T cells under controlled, consistent conditions with serum-free media supplements.
Analyze the T cell fraction: get cell counts and determine immune cell composition and transduction efficiency with flow characterization.
preservation
Determine the correct dosage using automated cell counting.
Products labelled as being manufactured under Good Manufacturing Practice (GMP) indicate that they are consistently produced and controlled according to quality requirements - it also means that products follow updated standards, with quality documentation and traceability, under independent QA oversight. BioLegend GMP products are manufactured in a dedicated GMP facility and are compliant with our ISO 13485:2016-certified quality management system. Our GMP products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products Ph. Eur. Chapter 5.2.12.
In addition to incorporating quality clinical-grade reagents throughout the manufacturing workflow, our schematic shows that robust phenotypic analyses are needed to monitor the purity, identity, and yield of immune cells at select points during the manufacturing process. For example, researchers can detect antigen-specific T cells at key steps of the workflow with Flex-T™ multimers pre-loaded with disease-relevant peptides and identify T cells using flow cytometry. To further profile T cells, it is useful to measure their secreted cytokines. Use ELISA and LEGEND MAX™ to quantitate levels of single cytokines secreted by T cells. Or use LEGENDplex™ multiplexed flow-based immunoassays to measure multiple cytokines in the same sample. It is also critical to discern the health status of processed cells, not only to measure cell viability but also to study the efficacy of tumor eradication therapies. You may find our optimized flow panels and resources on apoptosis and cell health among the helpful tools you need to evaluate final cell products.
The focus of this blog is cell manufacturing - from activation and culturing to phenotyping - with the ultimate goal of helping researchers scale up cell manufacturing processes. But we also understand that the journey starts with the discovery phase. Learn about the suite of C> tools offered by Revvity to help identify, validate, and optimize potential targets for therapeutic intervention. There you can explore advanced genomic technologies offered by Revvity, including proprietary base editing and CRISPR tools, to pinpoint and modify genes of interest. Together, BioLegend and Revvity offer C> researchers the knowledge and expertise to develop cell therapy strategies that make personalized and novel therapeutics possible in the arena of cancer treatments. Products mentioned here are for research use only and not for use in diagnostic procedures. For additional information, refer to our quality policies at BioLegend.
References
- Mukherjee, Anirban Goutam et al. “Role of Immune Cells and Receptors in Cancer Treatment: An Immunotherapeutic Approach.” Vaccines vol. 10,9 1493. 7 Sep. 2022, doi:10.3390/vaccines10091493. PubMed.
- Abou-El-Enein, Mohamed et al. “Scalable Manufacturing of CAR T cells for Cancer Immunotherapy.” Blood cancer discovery vol. 2,5 (2021): 408-422. doi:10.1158/2643-3230.BCD-21-0084. PubMed.
 Login / Register
Login / Register 








Follow Us