Activate and Expand T Cells for Bioprocessing With GMP Serum-Free Functional Antibodies
Cancer immunotherapies are revolutionizing cancer treatment by boosting the body’s own immune system to target and eliminate malignant tumors. T cells and their capacity for antigen-directed cytotoxicity play a central role, beginning with activation from two separate signals: its T cell receptor (TCR)-CD3 complex engaging antigen presenting cells (APCs) and the binding of co-stimulatory CD28 T cell receptor to either B7-1 (CD80) or B7-2 (CD86) proteins on APCs1.
Explore our resources to learn more about the most promising treatments2, such as adoptive cell therapies (ACT) like chimeric antigen receptor (CAR)-T or Natural Killer (NK) cells, monoclonal antibodies, and immune checkpoint inhibitors. In ACTs, T cells from a patient (autologous) or healthy donor (allogeneic) are activated and expanded in lab and then infused into the patient to treat the cancer. CAR-engineered T/NK cells more effectively recognize tumors and kill them in a targeted manner. While B cell malignancies and blood cancers are currently treated by CAR-T cells, the next generation CAR-T cells are being engineered to target solid tumors3, with CAR-NK cells promising to be an even safer, more effective cancer treatment4.
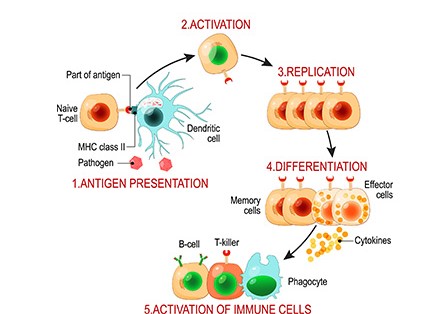
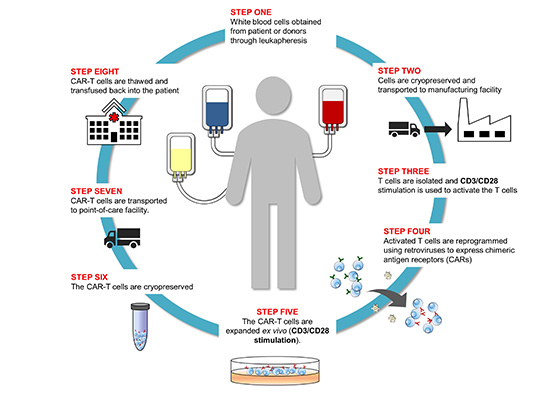
Image courtesy of Cell Culture Dish, www.CellCultureDish.com
For T cell therapies to be successful, a critical early step is the ex vivo activation and processing of T cells needed to generate a massive expansion of cells for downstream manufacturing of cell therapy products. As in an organism in vivo, ex vivo activation relies on dual stimulatory signals from anti-human CD3 and anti-human CD28 antibodies, followed by addition of peripheral blood mononuclear cell (PBMC)-derived T cells in culture media and incubation time to allow for T cell activation/expansion. Regardless of T cell activation approach —antibody-coated plates or soluble antibodies —the use of anti-human CD3 and anti-human CD28 antibodies to activate T cells is central to the T cell processing workflow. It follows then that the formulations and specifications of these activating antibodies are also critical.
As our recent article outlines, cell & gene therapies (CGTs) offer life-sustaining medicinal products, but face challenges in adopting robust and well-characterized biomanufacturing processes. Raw materials play an important, early role in controlling the overall quality, safety, and efficacy of the final products. For CGTs, raw materials are reagents, solvents, substances, or components used in the manufacturing process, but are not intended to be part of the final therapeutic product. A common issue is variability in raw materials that, in turn, could lead to underperformance and batch failures.
Adopting raw materials for CGT manufacturing that comply with regulatory requirements, including GMP and other quality standards, goes a long way to ensure biomanufacturers can properly evaluate the suitability of materials for their own process. Overall, best practices recommended by regulatory experts is for biomanufacturers to use GMP-grade and animal-derived component-free (ADCF) raw materials, procured from safe and traceable sources to achieve the most control and consistency in their process. This reduces potential roadblocks to a scalable supply chain and smooths the transfer path between research, clinical, and manufacturing partners5.
Animal-derived components, such as fetal bovine serum (FBS), are heterogeneous in nature, which negatively impacts product consistency5. For T cell activation procedures, BioLegend manufactures monoclonal antibodies Cell-Vive™ GMP Ultra-LEAF™ Purified anti-human CD3 SF Antibody (clone OKT3) and GMP Ultra-LEAF™ Purified anti-human CD28 SF Antibody (clone S20013F) under serum-free (SF) conditions, from start to finish. We also offer biotinylated formats, such as GMP Ultra-LEAF™ Biotin anti-human CD3 SF Antibody and Cell-Vive™ GMP Ultra-LEAF™ Biotin anti-human CD28 SF Antibody, ideal as probes to facilitate T cell research or T cell separation .
Our CD3 and CD28 antibodies are optimized for T cell activation and expansion in ex vivo cell culture applications.
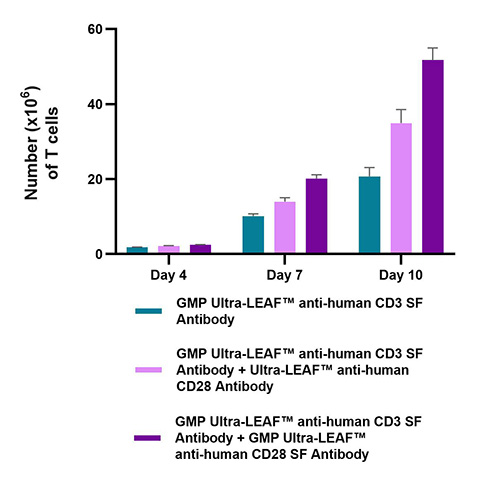
PBMC-derived T cells were activated in the presence of GMP Ultra-LEAF™ anti-human CD3 antibody (clone OKT3) alone or combined with Ultra-LEAF™ anti-human CD28 or GMP Ultra-LEAF™ anti-human CD28 SF antibody (both clone S20013F) and cultured for a total of 10 days. Our functional data clearly show the GMP Ultra-LEAF™ anti-human CD28 SF antibody yields superior T cell numbers.
We source the highest quality raw materials to manufacture these SF antibodies, thus removing risk while providing additional control, consistency, and safety for T cell-based workflows.
BioLegend GMP Ultra-LEAF™ SF antibody specifications:
- Purification by affinity chromatography under azide-free conditions and filtration through a 0.1 µm membrane.
- QC testing to be bioburden negative.
- Ultra-low in endotoxin (<0.01 EU/µg of protein).
- Validation testing by flow cytometry.
- Ultra-LEAF™ formulation which comes with quality testing by functional assays.
- Manufactured in a dedicated GMP facility that is ISO 13485:2016 and MDSAP certified, monitored through independent QA oversight.
- Manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12.
- Complete raw material traceability and documentation support.
- DMF support will be available.
By following GMP, BioLegend manufactures these CD3 and CD28 antibodies under standardized conditions and processes governed by quality oversight. This ensures they are produced in a controlled manner. In addition, we source the highest quality raw materials to manufacture all of our GMP SF bioprocessing reagents, thus helping to reduce batch-to-batch variability and improving the overall consistency of the final product. As a partner to biomanufacturers, we understand the importance of being a reliable and trustworthy supplier of high-quality materials that are manufactured in GMP-certified facilities. This ultimately reduces the burden of qualification on CGT manufacturers, facilitating translation from research to later stage development and marketing6.
Our scientists recognize the current challenges for the cell therapy industry. In addition to GMP SF functional CD3 and CD28 antibodies, we offer novel SF and chemically-defined GMP cell culture reagents to achieve controlled cell culture environments for the expansion, activation, and differentiation of T cells. Biomanufacturers can make use of our non-GMP solutions that can be implemented across the T cell therapy workflow to seamlessly transition from R&D to clinical applications. We also provide custom GMP development and manufacturing services for antibodies and ancillary reagents based on customer-specific program requirements. As your trusted partner, we want to enable your research so it leads to greater insights to T cell development and function, leading to improved therapeutics. Explore our entire workflow solutions.
References
- Xia, Fan et al. “TCR and CD28 Concomitant Stimulation Elicits a Distinctive Calcium Response in Naive T Cells.” Frontiers in immunology vol. 9 2864. 4 Dec. 2018, doi:10.3389/fimmu.2018.02864. PubMed.
- Mukherjee, Anirban Goutam et al. “Role of Immune Cells and Receptors in Cancer Treatment: An Immunotherapeutic Approach.” Vaccines vol. 10,9 1493. 7 Sep. 2022, doi:10.3390/vaccines10091493. PubMed.
- Reynolds, Sharon. “Engineering Car T-Cell Therapy to Overcome Limitations.” National Cancer Institute, NIH, 8 Feb. 2023, https://www.cancer.gov/news-events/cancer-currents-blog/2023/car-t-cell-therapies-overcoming-limitations.
- Li, Hongwen et al. “Preclinical and clinical studies of CAR-NK-cell therapies for malignancies.” Frontiers in immunology vol. 13 992232. 24 Oct. 2022, doi:10.3389/fimmu.2022.992232. PubMed.
- Bhattacharya, Sam, and Aparna Telang. “Manufacturing and Qualification Challenges for Cell and Gene Therapy Products and Best Practices for Success.” Cell Culture Dish, BioLegend, 6 Mar. 2023, https://cellculturedish.com/manufacturing-and-qualification-challenges-for-cell-and-gene-therapy-products-and-best-practices-for-success/.
- Tsokas, Katherine et al. “Reducing Risks and Delays in the Translation of Cell and Gene Therapy Innovations into Regulated Products.” NAM perspectives vol. 2019 10.31478/201909d. 30 Sep. 2019, doi:10.31478/201909d. PubMed.

 Login / Register
Login / Register 






Follow Us