- Know your cytometer configuration to define how many colors can be used in a single panel.
Most advanced cytometers have 5 lasers including ultraviolet (355 nm), violet (405 nm), blue (488 nm), green (532 nm) or yellow-green (561 nm), and red (640 nm). In addition to the lasers, there are a limited number of PMT (photomultiplier tube) detectors and filter combinations that determine the configuration of the cytometer. Together, this configuration will define how many and which parameters or colors can be detected simultaneously in one panel. Please note, most cytometers are highly customizable, especially those used for research purposes, and not all cytometers of given model have identical configuration. If you can't identify configuration of your specific cytometer, please contact your core facility manager who can help you to find this information.
- Perform a literature search to identify the essential markers for populations of interest.
A good panel is defined by its ability to resolve populations of interest. Therefore, make sure to include proper phenotyping and functional markers that suit your study goal. You can start your journey with these helpful links: Essential Markers; Maturation Markers; Cell Markers. Also, pay attention to biology of your sample, i.e. how well is the population of interest represented; autofluorescence of the cells; how temperature, stimulus, and enzymatic treatment affects the expression of surface markers on the population of interest. To learn more about how sample biology affects flow cytometry staining, read our next blog.
- Determine Antigen/Fluorophore combination.
When using several colors, compromises may have to be made due to availability of spectrally distinct fluorophores, antibodies and flow cytometer configuration. However, try to follow these rules:
- Match brightest fluorophores with antigens of the lowest expression level.
- Assign dimmer fluorophores to antigens with abundant expression. Use the Fluorophore Brightness Index to guide your decision.
- Assign synthetic fluorophores to intracellular antigens such as cytokines and transcriptional factors. Synthetic flourophores (i.e. Alexa Fluor® and Brilliant Violet™) are smaller than protein fluorophores (i.e. PE or APC) and tandem dyes (i.e. PE/Cy7 or APC/Cy7). Using antibodies conjugated to synthetic dyes increase chances of their delivery to subcellular compartments such as the nucleus.
- Use caution when choosing tandem dyes if fixing and permeabilizing cells is required. Some tandems may be more affected by long exposure to fixatives.
- Plan your gating strategy to limit comparisons of fluors with heavy spillover.
When the emission spectra of two fluorophores overlap, spillover of one fluorophore into the detection channel of the other might be observed. This is especially true for the tandem dyes using the same donor or acceptor (i.e. BV605™ and BV650™ use the same donor BV421™) or dyes that can be excited by more than one laser. In the case of PE/Cy5, donor (PE) is excited by the blue laser, while acceptor Cy5 is excited by the red laser, leading to direct overlap with APC which is excited by the red laser and has similar spectra overlap with Cy5. (Fig.1). Take this into account when building a panel by spreading markers across multiple lasers, using fluorophores with little or no overlap for cell subset markers (i.e. T cell subsets) and using closely overlapping fluorophores for mutually exclusive markers (such as CD3 and CD19). To check emission spectra overlap between fluorophores of your choice, use our Fluorescence Spectra Analyzer.
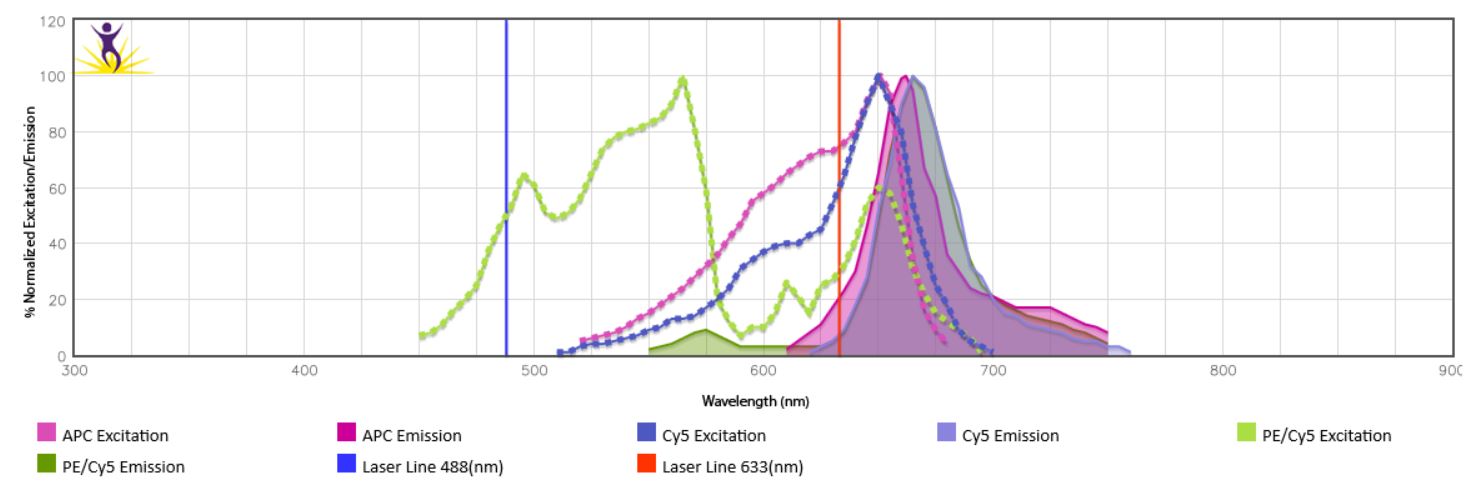
Figure 1. Spectra overlap between PE/Cy5 and APC.
- Use our Multicolor Panel Selector/Panel Builder to build a panel based on configuration and markers of your choice.
Also, this amazing web tool will provide available fluorophore-conjugated antibodies for your target and allow you to easily construct your panel and choose antibody combinations. The full tutorial to the multicolor selector can be found here. We also offer a Panel Builder powered by FluoroFinder. You can build a panel using preset instrument configurations based on actual instrumentation available at your facility or select your flow cytometer of choice. It also has a built in Spectra Analyzer that you can use while building the panel. Once you've built your panel with either tool, you can add items to your shopping cart, export/print the data, or email to a colleague.
- Don't forget to include Live/Dead discrimination dyes.
Dead cells often result in false positive events due to high autofluorescence and increased non-specific binding to antibodies . Not all dead cells can be easily gated by forward (FSC) and side scatter (SSC). Therefore, use of Live/Dead discrimination dyes is essential for your study, especially when doing intracellular staining. BioLegend offers a wide variety of Live Cell/Dead Cell Discrimination dyes. Please note, DNA binding dyes such as Helix NP™, DRAQ7™, Propidium Iodide and 7-AAD are not compatible with fixation. If you plan to fix your samples, use Zombie dyes instead.
|
 Login / Register
Login / Register 







Follow Us