- Clone
- W6/32 (See other available formats)
- Other Names
- Major Histocompatibility Class I, MHC class I
- Isotype
- Mouse IgG2a, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
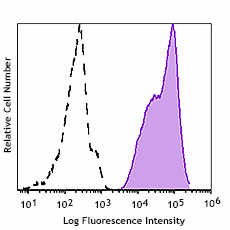
-

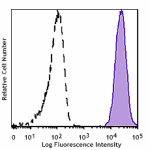
Human peripheral leukocytes were stained with GMP Ultra-LEAF™ Purified anti-human HLA-A,B,C SF antibody (Clone W6/32, filled histogram) or isotype control (Clone MOPC-173, open histogram) followed by anti-mouse IgG FITC stain.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 311460 | 1 mg | 1584€ | ||||
| 311459 | 100 µg | 308€ | ||||
MHC class I antigens associated with β2-microglobulin are expressed by all human nucleated cells. MHC class I molecules are involved in presentation of antigens to CD8+ T cells. They play an important role in cell-mediated immune responses and tumor surveillance. GMP Ultra-LEAF™ Purified anti-human HLA-A,B,C SF Antibody was GMP manufactured under serum-free conditions including serumfree hybridoma cell culture, without additional animal or human-derived materials or preservatives. This antibody contains ultra-low levels of endotoxin (<0.01EU/μg of protein), is filtered through a 0.1 μm membrane, and is tested negative for mycoplasma and microbial growths. GMP Ultra-LEAF™ Purified anti-human HLA-A,B,C SF Antibody is for research and further ex vivo bioprocessing use only
Product DetailsProduct Details
- Reactivity
- Human
- Reported Reactivity
- African Green, Baboon, Cat, Cow, Chimpanzee, Cynomolgus, Rhesus
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- 0.1 μm filtered in phosphate-buffered solution, pH 7.2, containing no preservative. Endotoxin level is < 0.01 EU/μg of the protein (< 0.001 ng/μg of the protein) as determined by the LAL test
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- 1.0 mg/mL
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
FC - Quality tested
Activ, Block, IHC-F, IP, WB - Reported in the literature, not verified in house
- Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 2.0 µg per million cells in 100 µL volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Clone W6/32 recognizes residues in the N terminus of the human ß2-microglobulin molecule21.
Additional reported applications (for the relevant formats) include: immunoprecipitaton2, Western blotting (non-reducing)3, immunohistochemical staining of acetone-fixed frozen tissue sections4,5, blocking6,7, inhibition of NK cell-mediated lysis10, and activation8,9. Clone W6/32 has been reported not to be suitable for immunohistochemistry on paraffin sections17. - Additional Product Notes
-
This GMP Ultra-LEAF™ Purified antibody (Endotoxin < 0.01 EU/μg, Azide-Free, 0.1 μm filtered) is recommended for highly sensitive assays. The antibody was GMP manufactured under serum-free conditions including serum-free hybridoma cell culture, without additional animal or human-derived materials or preservatives. This antibody is intended for flow cytometry and for research or further ex vivo bioprocessing use only.
-
Application References
(PubMed link indicates BioLegend citation) -
- Darrow TL, et al. 1989. J. Immunol. 142:3329.
- Stern P, et al. 1987. J. Immunol. 138:1088.
- Tran TM, et al. 2001. Immunogenetics 53:440.
- Barbatis C, et al. 1981. Gut 22:985.
- Ayyoub M, et al. 2004. Cancer Immunity 4:7.
- DeFelice M, et al. 1990. Cell. Immunol. 126:420.
- Fayen J, et al. 1998. Int. Immunol. 10:1347.
- Turco MC, et al. 1988. J. Immunol. 141:2275.
- Geppert TD, et al. 1989. J. Immunol. 142:3763.
- Wooden SL, et al. 2005. J. Immunol. 175:1383.
- Nagano M, et al. 2007. Blood 110:151.
- McLoughlin RM,et al.2008. J. Immunol. 181:1323. PubMed
- Takahara M, et al.2008. J. Leukoc. Biol. 83:742. PubMed
- Lunemann A, et al.2008. J. Immunol. 181:6170. PubMed
- Laing BJ, et al. 2010. J. Thorac Cardiovasc Surg. 139:1402. PubMed
- Yoshino N, et al. 2000. Exp. Anim. (Tokyo) 49:97. (FC)
- Vambutas A, et al. 2000. Clin. Diagn. Lab. Immun. 7:79.
- Coppieters KT, et al. 2012. J. Exp. Med. 209:51. (epitope)
- Crivello P, et al. 2013. Hum Immunol. 22:100. PubMed
- Jung Y, et al. 2015. Mol Cancer Res. 13:197. PubMed
- Shields MJ. Ribaudo RK. 1998. Tissue Antigens. 51(5):567-70. (epitope)
- Disclaimer
-
GMP Ultra-LEAF™ antibodies. BioLegend GMP Ultra-LEAF™ antibodies are manufactured in a dedicated GMP facility and compliant with ISO 13485:2016. For research or ex vivo cell processing use. Not for use in diagnostic or therapeutic procedures. Our processes include:
- Batch-to-batch consistency
- Material traceability
- Documented procedures
- Documented employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- Quality audits per ISO 13485:2016
- QA review of released products
BioLegend GMP Ultra-LEAF™ antibodies are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12
Antigen Details
- Structure
- Ig superfamily
- Distribution
-
All nucleated cells
- Function
- Antigen presentation
- Ligand/Receptor
- CD3/TCR, CD8
- Biology Area
- Immunology, Innate Immunity
- Molecular Family
- MHC Antigens
- Antigen References
-
1. Barclay AN, et al. Eds. 1993. The Leukocyte Antigen FactsBook. Academic Press Inc. San Diego.
- Gene ID
- 3105 View all products for this Gene ID
- UniProt
- View information about HLA-A on UniProt.org
Related Pages & Pathways
Pages
Other Formats
View All HLA-A,B,C Reagents Request Custom ConjugationCompare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
APC anti-human HLA-A,B,C
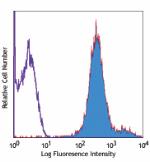
Human peripheral blood lymphocytes stained with W6/32 APC -
FITC anti-human HLA-A,B,C
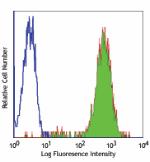
Human peripheral blood lymphocytes stained with W6/32 FITC -
PE anti-human HLA-A,B,C
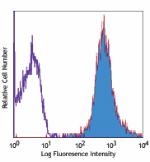
Human peripheral blood lymphocytes stained with W6/32 PE -
PE/Cyanine5 anti-human HLA-A,B,C
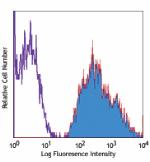
Human peripheral blood lymphocytes stained with W6/32 PE/Cya... -
Purified anti-human HLA-A,B,C
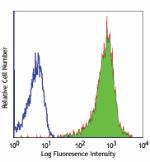
Human peripheral blood lymphocytes were stained with purifie... 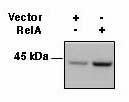
HEK293 cells were transfected with RelA or empty vector and ... -
Alexa Fluor® 488 anti-human HLA-A,B,C
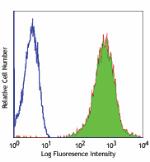
Human peripheral blood lymphocytes stained with W6/32 Alexa ... -
Alexa Fluor® 647 anti-human HLA-A,B,C
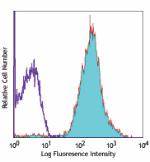
Human peripheral blood lymphocytes stained with W6/32 Alexa ... -
Pacific Blue™ anti-human HLA-A,B,C
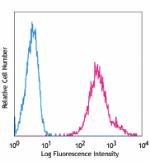
Human peripheral blood lymphocytes stained with W6/32 Pacifi... -
PerCP anti-human HLA-A,B,C
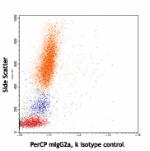
Human peripheral blood lymphocytes, monocytes and granulocyt... 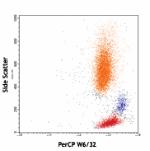
-
APC/Cyanine7 anti-human HLA-A,B,C
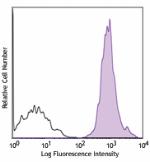
Human peripheral blood lymphocytes were stained with HLA-A,B... -
PerCP/Cyanine5.5 anti-human HLA-A,B,C
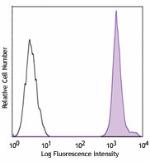
Human peripheral blood lymphocytes were stained with HLA-A,B... -
Ultra-LEAF™ Purified anti-human HLA-A,B,C
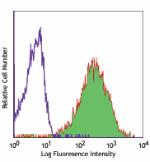
Human peripheral blood lymphocytes stained with Ultra-LEAF™ ... -
PE/Cyanine7 anti-human HLA-A,B,C
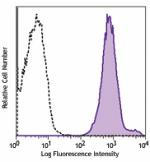
Human peripheral blood lymphocytes were stained with HLA-A,B... -
Brilliant Violet 510™ anti-human HLA-A,B,C
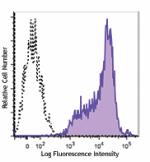
Human peripheral blood lymphocytes were stained with HLA-A, ... -
Alexa Fluor® 700 anti-human HLA-A,B,C
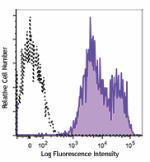
Human peripheral blood lymphocytes were stained with HLA-A,B... -
PE/Dazzle™ 594 anti-human HLA-A,B,C
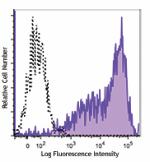
Human peripheral blood lymphocytes were stained with HLA-A,B... -
Biotin anti-human HLA-A,B,C
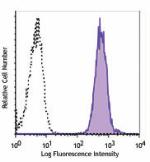
Human peripheral blood lymphocytes were stained with biotiny... -
Brilliant Violet 605™ anti-human HLA-A,B,C
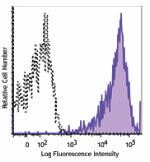
Human peripheral blood lymphocytes were stained with HLA-A,B... -
APC/Fire™ 750 anti-human HLA-A,B,C
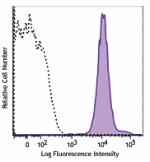
Human peripheral blood lymphocytes were stained with HLA-A,B... -
TotalSeq™-A0058 anti-human HLA-A,B,C
-
TotalSeq™-C0058 anti-human HLA-A,B,C
-
TotalSeq™-B0058 anti-human HLA-A,B,C
-
Spark NIR™ 685 anti-human HLA-A,B,C
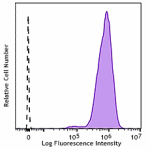
Human peripheral blood lymphocytes were stained with HLA-A,B... -
TotalSeq™-D0058 anti-human HLA-A,B,C
-
GMP Ultra-LEAF™ Purified anti-human HLA-A,B,C SF
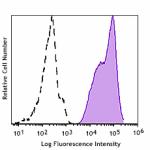
Human peripheral leukocytes were stained with GMP Ultra-LEAF... -
GMP Ultra-LEAF™ Biotin anti-human HLA-A,B,C SF
Human peripheral leukocytes were stained with GMP Ultra-LEAF... -
Spark UV™ 387 anti-human HLA-A,B,C

Human peripheral blood lymphocytes were stained with anti-hu... 
Human peripheral blood lymphocytes were stained anti-human H... -
Spark Red™ 718 anti-human HLA-A,B,C (Flexi-Fluor™)
-
Spark Blue™ 574 anti-human HLA-A,B,C (Flexi-Fluor™)
-
Spark Blue™ 550 anti-human HLA-A,B,C (Flexi-Fluor™)
 Login / Register
Login / Register 








Follow Us