- Clone
- 4G8 (See other available formats)
- Regulatory Status
- RUO
- Workshop
- HCDM listed
- Other Names
- AAA, ABETA, ABPP, AD1, APPI, CTFgamma, CVAP, PN-II, PN2, Amyloid beta A4 protein, preA4, protease nexin-II, peptidase nexin-II, beta-amyloid peptide, alzheimer disease amyloid protein, cerebral vascular amyloid peptide, APP, Amyloid Precursor Protein
- Isotype
- Mouse IgG2b, κ
- Ave. Rating
- Submit a Review
- Product Citations
- 2 publications
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 800719 | 25 µg | 95€ | ||||
| 800720 | 100 µg | 249€ | ||||
Alzheimer’s disease is characterized by the accumulation of aggregated Aβ peptides in senile plaques and vascular deposits. Aβ peptides are derived from amyloid precursor proteins (APP) through sequential proteolytic cleavage of APP by β-secretases and γ-secretases generating diverse Aβ species. Aβ can aggregate to form soluble oligomeric species and insoluble fibrillar or amorphous assemblies. Some forms of the aggregated peptides are toxic to neurons.
Product DetailsProduct Details
- Verified Reactivity
- Human, Mouse
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- This antibody is provided in 50% glycerol in aqueous buffered solutions with preservatives.
- Preparation
- The antibody was purified by affinity chromatography and conjugated with HRP under optimal conditions.
- Storage & Handling
- Upon receipt, the antibody solution should be stored undiluted at -20°C, and protected from prolonged exposure to light.
- Application
-
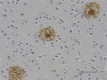
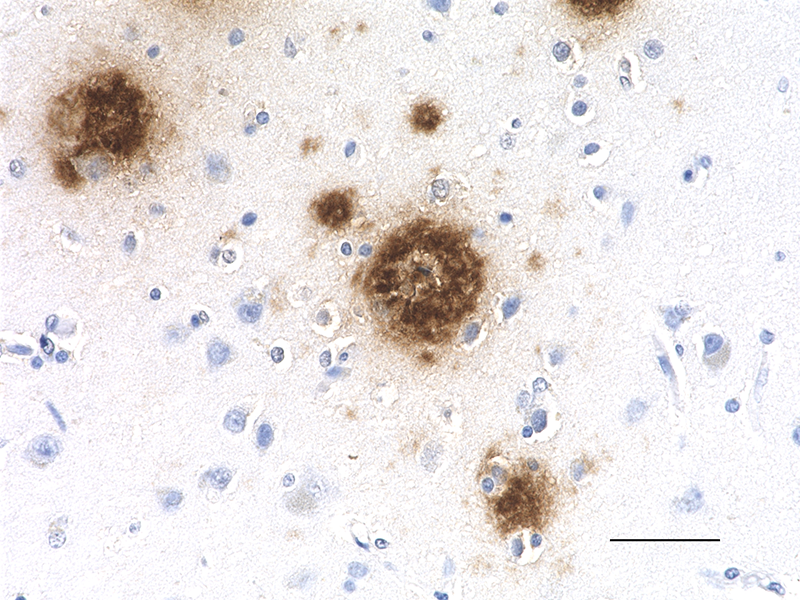
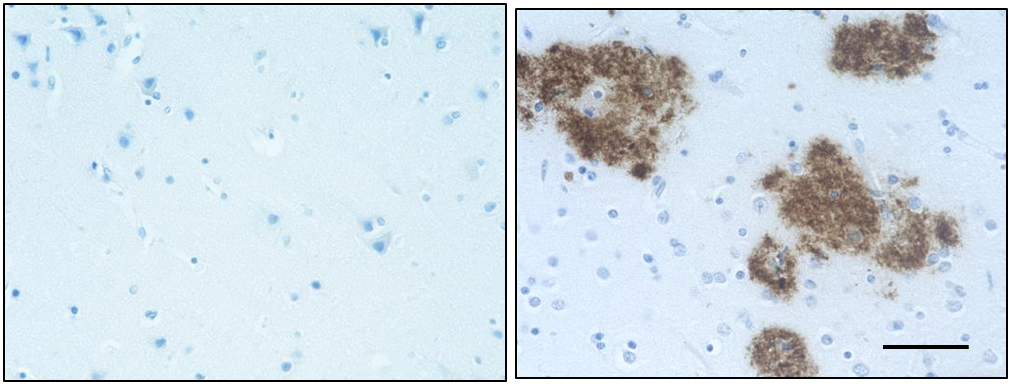
IHC-P - Quality tested
- Recommended Usage
-
Each lot of this antibody is quality control tested by formalin-fixed paraffin-embedded immunohistochemical staining. For immunohistochemistry, a concentration range of 1.0 - 5.0 µg/ml is suggested. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
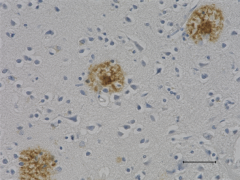
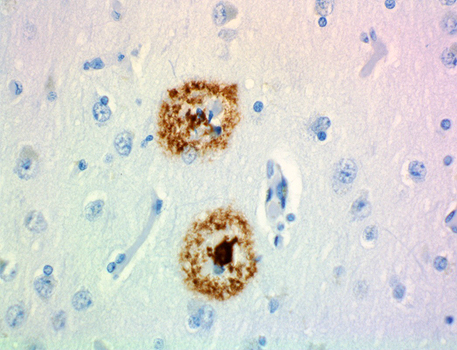
This antibody is reactive to amino acid residues 17-24 of ß amyloid. The epitope lies within amino acids 18-22 of ß amyloid (VFFAE). 4G8 ß-amyloid antibody reacts to abnormally processed isoforms, as well as precursor forms.
This antibody clone has been reported for use on IHC of free-floating sections in PBS containing 1% Triton incubated with 0.1 m citrate buffer (4).
Additional reported applications (for the relevant formats) include: immunohistochemical staining on frozen tissue sections (IHC-F) and immunocytochemistry (ICC) -
Application References
(PubMed link indicates BioLegend citation) -
- Poduslo JF, et al. 2004. Biochem. 43:6064. (IHC-F) PubMed
- Forny-Germano L, et al. 2014. J Neurosci. 34:13629. (IHC-Other) PubMed
- Vallino Costassa E, et al. 2016. J Alzheimers Dis. 51: 875-87. (IHC-P) PubMed
- Chen X, et al. 2013. Neurobiol Aging. 34:2370. (ICC) PubMed
- Hatami A, et al. 2016. J Alzheimers Dis. 50:517. (IHC-P) PubMed
- Kawarabayashi T, et al. 2001. J Neurosci 21:372 (IP)
- Fonte V, et al. 2002. PNAS. 110:4853 (IP)
- Product Citations
-
- RRID
-
AB_2783371 (BioLegend Cat. No. 800719)
AB_2783371 (BioLegend Cat. No. 800720)
Antigen Details
- Structure
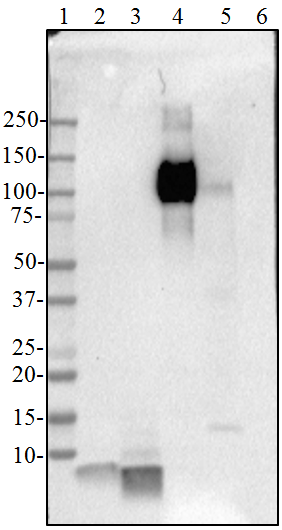
- Amyloid precursor protein is a 770 amino acid protein with a molecular mass of ~100 kD. According to the UniProtKB database, APP (ID# P05067) has 11 isoforms (34 to ~90 kD) and the 770 form has been designated as the canonical form. Isoform APP695 is the predominant form expressed in neuronal tissue. Isoforms APP751 and APP770 are widely expressed in non-neuronal cells. Isoform APP751 is the most abundant form in T-lymphocytes. Aβ denotes peptides of 36-43 amino acids generated from cleavage of APP by secretases. Aβ has an apparent molecular mass of about 4 kD.
- Distribution
-
Tissue distribution: Primarily nervous system, but also adipose tissue, intestine, and muscle.
Cellular distribution: Cytosol, endosomes, nucleus, plasma membrane, extracellular, and golgi apparatus. - Function
- The normal function of Aβ is not well understood. Several potential physiological roles have been proposed, including: activation of kinase enzymes; protection against oxidative stress; regulation of cholesterol transport; transcription factor, and as an anti-microbial agent.
- Biology Area
- Cell Biology, Neurodegeneration, Neuroscience, Protein Misfolding and Aggregation
- Molecular Family
- APP/β-Amyloid
- Antigen References
-
- Kumar A, et al. 2015. Pharmacol Rep. 67(2):195.
- Sadigh-Eteghad S, et al. 2015. Med Princ Pract. 24(1):1
- Hampel H, et al. 2015. Expert Rev Neruother. 15(1):83.
- Puig KL, et al. 2012. Exp Gerontol. 48(7): 608.
- Selkoe DJ, et al. 2016. EMBO Mol Med. 8(6):595.
- Walsh DM, et al. 2007. J Neurochem. 101(5):1172.
- Gene ID
- 351 View all products for this Gene ID
- UniProt
- View information about beta-Amyloid 17-24 on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
Other Formats
View All β-Amyloid, 17-24 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Anti-β-Amyloid, 17-24 | 4G8 | IHC-P,Direct ELISA,IHC-F,ICC |
| Biotin anti-β-Amyloid, 17-24 | 4G8 | IHC-P,Direct ELISA |
| Purified anti-β-Amyloid, 17-24 | 4G8 | IHC-P,Direct ELISA,IHC-F,ICC,IP |
| Alexa Fluor® 488 anti-β-Amyloid, 17-24 | 4G8 | IHC-P |
| Alexa Fluor® 647 anti-β-Amyloid, 17-24 | 4G8 | IHC-P |
| Alexa Fluor® 594 anti-β-Amyloid, 17-24 | 4G8 | IHC-P |
| HRP anti-β-Amyloid, 17-24 | 4G8 | IHC-P |
| Spark YG™ 570 anti-β-Amyloid, 17-24 | 4G8 | IHC-P |
Customers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Anti-β-Amyloid, 17-24
IHC staining of anti-β-Amyloid, 17-24 antibody (clone 4G8) o... 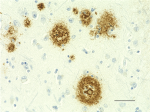
IHC staining of anti-β-Amyloid, 17-24 antibody (clone 4G8) o... 
Direct ELISA of anti-β-Amyloid, 17-24 (clone 4G8) antibody b... -
Biotin anti-β-Amyloid, 17-24
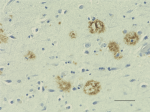
IHC staining of Biotin anti-β-Amyloid, 17-24 antibody (clone... 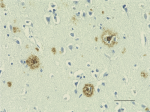
IHC staining of Biotin anti-β-Amyloid, 17-24 antibody (clone... 
Direct ELISA of Biotin anti-β-Amyloid, 17-24 (clone 4G8) ant... -
Purified anti-β-Amyloid, 17-24
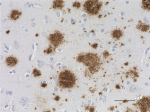
IHC staining of purified anti-β-Amyloid, 17-24 antibody (clo... 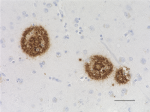
IHC staining of purified anti-β-Amyloid, 17-24 antibody (clo... 
Direct ELISA of purified anti-β-Amyloid, 17-24 (clone 4G8) a... -
Alexa Fluor® 488 anti-β-Amyloid, 17-24

IHC staining of Alexa Fluor® 488 anti-β-Amyloid, 17-24 antib... -
Alexa Fluor® 647 anti-β-Amyloid, 17-24
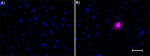
IHC staining of Alexa Fluor® 647 anti-β-Amyloid, 17-24 antib... -
Alexa Fluor® 594 anti-β-Amyloid, 17-24

IHC staining of Alexa Fluor® 594 anti-β-Amyloid, 17-24 antib... -
HRP anti-β-Amyloid, 17-24
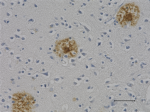
IHC staining of HRP anti-β-Amyloid, 17-24 antibody (clone 4G... -
Spark YG™ 570 anti-β-Amyloid, 17-24
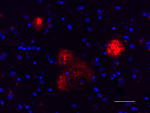
IHC staining of Spark YG™ 570 anti-β-Amyloid, 17-24 antibody...


 Login / Register
Login / Register 











Follow Us