- Regulatory Status
- RUO
- Other Names
- IBP4, BP-4, HT29-IGFBP, IGFBP4
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

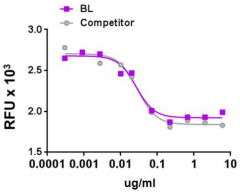
Recombinant human IGFBP-4 inhibits the proliferation of human mammary gland MCF-7 cell line induced by IGF II (18 ng/mL) in a dose dependent manner. BioLegend’s protein was compared side-by-side to a competitor’s equivalent product.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 750604 | 25 µg | 278€ | ||||
| 750606 | 100 µg | 713€ | ||||
Human IGFBP-4 was initially cloned from the human placenta, liver, and ovary cDNA libraries. Seven IGFBPs has been described that modulate the IGF activity. IGFBPs structurally are characterized by three domains: the amino-terminal, the carboxyl-terminal, and an intermediate variable L-domain. Some IGFBPs bind to the extracellular matrix (IGFBP-2, -3, and -5) or the cell membrane (IGFBP-1, -2, -3, and -5). The IGF binding activity of IGFBP-4 is mainly localized in the N-terminal region. IGFBP-4 is the smallest IGFBP, and there is no evidence for cell surface or ECM association of IGFBP-4. In addition, it contains an N-linked glycosylation site and exists in biological fluids as a doublet of 24 kD nonglycosylated and a 28 kD glycosylated forms. IGFBP-4 binds both IGF-I and IGF-II with similar affinities. Nevertheless, it is generally coexpressed with IGF-II during development. Proteolysis is the main regulatory mechanism of IGFBP-4 activity. Pregnancy-associated plasma protein-A (PAPP-A) was identified as an IGF-dependent IGFBP-4 protease. It belongs to the metzincin superfamily of metalloproteinases and cleaves IGFBP-4 at a single site. IGFBP-4 has potent IGF-independent anti-angiogenic and antitumorigenic effects, activities that are located in the C-terminal, and a region containing a thyroglobulin type 1 (Tg1) domain.
Product DetailsProduct Details
- Source
- Human IGFBP-4, amino acids (Asp22-Glu258) (Accession# AAA62670), was expressed in 293E cells. The amino-terminal possesses a 9His-tag.
- Molecular Mass
- The 259 amino acid recombinant protein has a predicted molecular mass of approximately 28 kD. The DTT-reduced and non-reduced protein migrates approximately at 35 - 40 and 33 - 38 kD by SDS-PAGE respectively. The predicted N-terminal amino acid is His.
- Purity
- >90%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in PBS pH 7.2.
- Endotoxin Level
- Less than 0.01 ng per µg cytokine as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 15 - 90 ng/ml as determined by the inhibition of MCF-7 cell proliferation induced by human IGF-II (18 ng/ml, Cat No. 590604).
- Application
-
Bioassay
- Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Distribution
-
It is expressed in a variety of tissues and cells such as embryonic and postnatal brain, human osteoblast-like cells, skin fibroblasts, endothelial cells, epithelial cells, intestinal epithelium, and in a variety of tumor cells.
- Function
- IGFBP-4 inhibits the action of IGFI and II by preventing their binding to their receptors. The binding affinity of IGFBP-4 for IGFs is regulated by proteolysis through PAPP-A.
- Ligand/Receptor
- IGF-I and IGF-II.
- Bioactivity
- IGFBP-4 inhibits the proliferation of MCF-7 cells induced by IGF-II (18 ng/ml).
- Cell Type
- Hematopoietic stem and progenitors, Mesenchymal Stem Cells
- Biology Area
- Angiogenesis, Cancer Biomarkers, Cell Biology, Cell Proliferation and Viability, Stem Cells
- Antigen References
-
1. Shimasaki S, et al. 1990. Molecular Endocrinol. 4:1451.
2. Boldt HB, et al. 2001. Biochem. J. 358:359.
3. Bunn RC and Fowlkes JL. 2003. Trends Endocrinol. Metab. 14:176.
4. Ning Y, et al. 2008. Mol. Endocrinol. 22:1213.
5. Moreno MJ, et al. 2013. Neoplasia. 5:554.
6. Contois LW, et al. 2012. J. Biol. Chem. 287:1779.
7. Shi Z, et al. 2013. Am. J. Physiol. Gastrointest. Liver Physiol. 305:G74. - Gene ID
- 3487 View all products for this Gene ID
- UniProt
- View information about IGFBP-4 on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 








Follow Us