- Clone
- 3F9 (See other available formats)
- Regulatory Status
- RUO
- Workshop
- VII 70502
- Other Names
- IL-10 Receptor, IL-10R
- Isotype
- Rat IgG2a, κ
- Ave. Rating
- Submit a Review
- Product Citations
- 3 publications
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 308817 | 100 µg | 216€ | ||||
| 308818 | 1 mg | 572€ | ||||
CD210, also known as the IL-10 receptor, is a 90-110 kD protein expressed on T cells, B cells, NK cells, monocytes and macrophages. CD210 belongs to the class II cytokine receptor family which includes the IFN-γ receptor (CDw119), the IFN-α/β receptor (CD118) and tissue factor (CD142). The IL-10 receptor is involved in signal transduction by inducing phosphorylation of STAT1a and STAT3 and by inducing activation of Jak1 and Tyk.
Product DetailsProduct Details
- Verified Reactivity
- Human, Cynomolgus, Rhesus
- Reported Reactivity
- African Green, Baboon
- Antibody Type
- Monoclonal
- Host Species
- Rat
- Immunogen
- shIL-10R
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- Less than 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
FC - Quality tested
IP, Block - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 2.0 µg per million cells in 100 µl volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Clone 3F9 recognizes the IL-10-binding epitope of IL-10R1.8 Additional reported applications (for the relevant formats) include: immunoprecipitation1, in vitro blocking1-3 of human IL-10 binding to IL-10R. For most successful immunofluorescent staining results, it may be important to maximize signal over background by using a relatively bright fluorochrome-antibody conjugate (Cat. No. 308804) or by using a high sensitivity, three-layer staining technique (e.g., including a biotinylated anti-rat IgG second step), followed by SAv-PE (Cat. No. 405204). The Ultra-LEAF™ purified antibody (Endotoxin < 0.01 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for highly sensitive assays (Cat. No. 308817 and 308818).
-
Application References
(PubMed link indicates BioLegend citation) -
- Liu Y, et al. 1997. J. Immunol. 158:604. (Immunogen, IP, Block)
- Levings MK, et al. 2005. Blood 105:1162. (Block)
- Goodier MR, et al. 2000. J. Immunol. 165:139. (Block)
- Huang YH, et al. 2009. J. Leukoc. Biol. 86:273. PubMed
- Yoshino N, et al. 2000. Exp. Anim. (Tokyo) 49:97. (FC)
- Liu BS,et al. 2011. J Leukoc Biol. 89:981. PubMed
- Joffe M, et al. 2012. Int Immunol. 24:447. PubMed
- MacDonald KP, et al. 1999. J. Immunol. 163:5599. (epitope)
- Product Citations
-
- RRID
-
AB_2800804 (BioLegend Cat. No. 308817)
AB_2800804 (BioLegend Cat. No. 308818)
Antigen Details
- Structure
- Ig superfamily, class II cytokine receptor family, transmembrane glycoprotein, 90-110 kD
- Distribution
-
T cells and B cells, NK cells, monocytes, macrophages
- Function
- Activates STAT1, STAT3, Jak1, Tyk
- Ligand/Receptor
- IL-10
- Cell Type
- B cells, Macrophages, Monocytes, NK cells, T cells
- Biology Area
- Immunology
- Molecular Family
- CD Molecules, Cytokine/Chemokine Receptors
- Antigen References
-
1. Kotenko S. 2002. Cytokine Growth Factor Rev. 13:223.
2. Trinchieri G. 2003. Nat. Rev. Immunol. 3:133. - Gene ID
- 3587 View all products for this Gene ID
- UniProt
- View information about CD210 on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Other Formats
View All CD210 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| PE anti-human CD210 (IL-10 R) | 3F9 | FC |
| Purified anti-human CD210 (IL-10 R) | 3F9 | FC,IP,Block |
| APC anti-human CD210 (IL-10 R) | 3F9 | FC |
| PE/Cyanine7 anti-human CD210 (IL-10 R) | 3F9 | FC |
| Brilliant Violet 421™ anti-human CD210 (IL-10 R) | 3F9 | FC |
| Ultra-LEAF™ Purified anti-human CD210 (IL-10 R) | 3F9 | FC,IP,Block |
| TotalSeq™-D1170 anti-human CD210 (IL-10 R) | 3F9 | PG |
| TotalSeq™-A1170 anti-human CD210 (IL-10 R) | 3F9 | PG |
| TotalSeq™-B1170 anti-human CD210 (IL-10 R) | 3F9 | PG |
Customers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
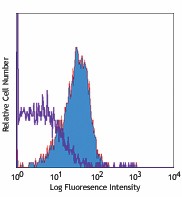
PE anti-human CD210 (IL-10 R)
Human peripheral blood lymphocytes stained with 3F9 PE -
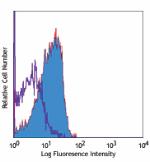
Purified anti-human CD210 (IL-10 R)
Whole blood lymphocytes stained with purified 3F9, then dete... -
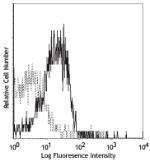
APC anti-human CD210 (IL-10 R)
Human peripheral blood lymphocytes were stained with CD210 (... -
PE/Cyanine7 anti-human CD210 (IL-10 R)
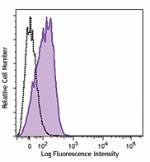
Human peripheral blood lymphocytes were stained with CD210 (... -
Brilliant Violet 421™ anti-human CD210 (IL-10 R)
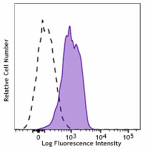
Human peripheral blood lymphocytes were stained with CD210 (... -
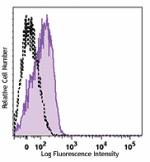
Ultra-LEAF™ Purified anti-human CD210 (IL-10 R)
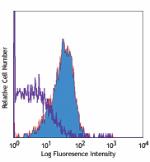
Human peripheral blood lymphocytes stained with LEAF™ purifi... -
TotalSeq™-D1170 anti-human CD210 (IL-10 R)
-
TotalSeq™-A1170 anti-human CD210 (IL-10 R)
-
TotalSeq™-B1170 anti-human CD210 (IL-10 R)


 Login / Register
Login / Register 















Follow Us