- Clone
- 17G10.2 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- CD85g, immunoglobulin-like transcript 7, LILRA4
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 326413 | 100 µg | 216€ | ||||
| 326414 | 1 mg | 595€ | ||||
ILT7 (immunoglobulin-like transcript 7) is a member of leukocyte immunoglobulin-like receptor (LIR or LILR) gene family, also known as CD85g and LILRA4. It contains four extracellular Ig domains. In association with FcεR1γ, ILT7 forms a receptor complex that transduces ITAM-mediated signals. ILT7 is specifically expressed on plasmacytoid dendritic cells (pDCs), but not on myeloid dendritic cells and other peripheral blood leukocytes. ILT7 negatively regulates the innate immune functions of human pDCs. Cross-linking 17G10.2 antibody is able to trigger ILT7 mediated signaling.
Product DetailsProduct Details
- Verified Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- Less than 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
FC - Quality tested
FA - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 0.5 µg per million cells in 100 µL volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Additional reported (for the relevant formats) applications include: trigger ILT7 mediated signaling.The Ultra-LEAF™ purified antibody (Endotoxin < 0.01 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for functional assays (Cat. No. 326413 and 326414).
-
Application References
(PubMed link indicates BioLegend citation) -
- Jensen MA, et al. 2013. Ann Rheum Dis. 72:596. PubMed.
- RRID
-
AB_2860854 (BioLegend Cat. No. 326413)
AB_2860854 (BioLegend Cat. No. 326414)
Antigen Details
- Structure
- Associate with FcεR1γ leukocyte immunoglobulin-like receptor gene family
- Distribution
-
Plasmacytoid dendritic cells
- Function
- Negatively regulates pDC mediated innate immune function
- Cell Type
- Dendritic cells
- Biology Area
- Immunology, Innate Immunity
- Molecular Family
- CD Molecules
- Antigen References
-
1. Cao W, et al. 2006. J. Exp. Med. 203(6):1399.
2. Brown D, et al. 2004. Tissue Antigen. 64:215.
3. Rissoan MC, et al. 2002. Blood 100:3295. - Gene ID
- 23547 View all products for this Gene ID
- UniProt
- View information about CD85g on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Other Formats
View All CD85g (ILT7) Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| PE anti-human CD85g (ILT7) | 17G10.2 | FC |
| Alexa Fluor® 647 anti-human CD85g (ILT7) | 17G10.2 | FC |
| Ultra-LEAF™ Purified anti-human CD85g (ILT7) | 17G10.2 | FC,FA |
| TotalSeq™-C0409 anti-human CD85g (ILT7) | 17G10.2 | PG |
| TotalSeq™-B0409 anti-human CD85g (ILT7) | 17G10.2 | PG |
| Brilliant Violet 421™ anti-human CD85g (ILT7) | 17G10.2 | FC |
| Spark Red™ 718 anti-human CD85g (ILT7) (Flexi-Fluor™) | 17G10.2 | FC |
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
PE anti-human CD85g (ILT7)
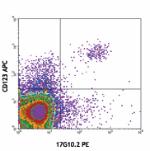
CD14- human peripheral blood mononuclear cells st... -
Alexa Fluor® 647 anti-human CD85g (ILT7)
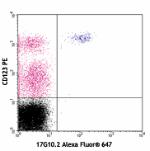
Human peripheral blood mononuclear cells stained with CD123 ... -
Ultra-LEAF™ Purified anti-human CD85g (ILT7)
-
TotalSeq™-C0409 anti-human CD85g (ILT7)
-
TotalSeq™-B0409 anti-human CD85g (ILT7)
-
Brilliant Violet 421™ anti-human CD85g (ILT7)

Human peripheral blood mononuclear cells were stained with a... -
Spark Red™ 718 anti-human CD85g (ILT7) (Flexi-Fluor™)

 Login / Register
Login / Register 









Follow Us