- Regulatory Status
- RUO
- Other Names
- Fixable Dye, Fixable Viability Dye
-

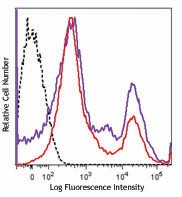
One day old C57BL/6 mouse splenocytes were stained with Zombie NIR™ and analyzed before fixation (purple) or after fixation and permeabilization (red). Cells alone, without Zombie NIR™ staining, are indicated in black.
| Cat # | Size | Price | Quantity Check Availability | ||
|---|---|---|---|---|---|
| 423105 | 100 tests | 72,00€ | |||
| 423106 | 500 tests | 268,00€ | |||
Zombie NIR™ is an amine reactive fluorescent dye that is non-permeant to live cells, but permeant to the cells with compromised membranes. Thus, it can be used to assess live vs. dead status of mammalian cells. Zombie NIR™ is a polar water soluble dye, providing red fluorescence, making it suitable for multi-color detection.
Product Details
- Preparation
- Zombie NIR™ Fixable Viability kit is composed of lyophilized Zombie NIR™ dye and anhydrous DMSO. For reconstitution, bring the kit to room temperature; add 100 µl of DMSO to one vial of Zombie NIR™ dye until fully dissolved. 100 tests = 1 vial of Zombie NIR™ + DMSO, 500 tests = 5 vials of Zombie NIR™ + DMSO.
- Storage & Handling
- Store kit at -20°C upon receipt. Do not open vials until needed. Once the DMSO is added to the Zombie NIR™ dye, use immediately, or store at -20°C in a dry place and protected from light, preferably in a desiccator or in a container with desiccant for no more than one month.
- Application
-
FC - Quality tested
- Recommended Usage
-
Each lot of this product is quality control tested by immunofluorescent staining with flow cytometric analysis.
For flow cytometry, the suggested dilution is 1:100-1:1000 for 1-10 million cells. It is recommended that the reagent be titrated for optimal performance for each application, as optimal dosage varies with cell type. - Excitation Laser
-
Red Laser (633 nm)
- Application Notes
-
Zombie NIR™ dye is excited by the red laser and has fluorescence emission maximum at 746 nm. If using in a multi-color panel design, filter optimization may be required depending on other fluorophores used. Zombie NIR™ dye has similar emission to APC/Cyanine7.
Standard Cell Staining Protocol:- Prior to reconstitution, spin down the vial of lyophilized reagent in a microcentrofuge to ensure the reagent is at the bottom of the vial.
- For reconstitution, pre-warm the kit to room temperature; add 100 µL of DMSO to one vial of Zombie NIR™ dye and mix until fully dissolved
- Wash cells with PBS buffer (no Tris buffer and protein free).
- Dilute Zombie NIR™ dye at 1:100-1000 in PBS. Resuspend 1-10 x 106 cells in diluted 100 µL Zombie NIR™ solution. To minimize background staining of live cells, titrate the amount of dye and/or number of cells per 100 µL for optimal performance. Different cell types can have a wide degree of variability in staining based on cell size and degree of cell death.
- Note: Don’t use Tris buffer as a diluent and be sure that the PBS does not contain any other protein like BSA or FBS.
- Note: The amount of dye used can also influence the ability to detect apoptotic as well as live and dead cells.
- Incubate the cells at room temperature (or 4°C), in the dark, for 15-30 minutes.
- Wash one time with 2 mL BioLegend’s Cell Staining Buffer (Cat. No. 420201) or equivalent buffer containing serum or BSA.
- Continue performing antibody staining procedure as desired.
- Cells can be fixed with paraformaldehyde or methanol prior to permeabilization or can be analyzed without fixation.
No-wash Sequential Staining Protocol:
- Wash cells with PBS buffer (no Tris buffer and protein free).
- For reconstitution, pre-warm the kit to room temperature; add 100 µL of DMSO to one vial of Zombie NIR™ dye and mix until fully dissolved
- Determine the total µL volume of antibody cocktail previously titrated and optimized for the assay that will be added to each vial/well of cells based on a final volume of 100 µL. Subtract that antibody volume from the 100 µL total staining volume intended for the assay. In the remaining volume, dilute Zombie NIR™ dye at 1:100-1000 in PBS as determined by prior optimization at that volume. For example, if you are adding 20 µL of antibody cocktail for a 100 µL total staining volume, use 80 µL of Zombie NIR™ solution. Resuspend 1-10 x 106 cells in the appropriate volume of Zombie NIR™ solution. Different cell types can have a wide degree of variability in staining based on cell size and degree of cell death.
- Note: Don’t use Tris buffer as a diluent and be sure that the PBS does not contain any other protein like BSA or FBS.
- Note: The amount of dye used can also influence the ability to detect apoptotic as well as live and dead cells.
- Incubate for 10-15 minutes at RT (or 4°C), protected from light. Without washing the cells, add the cell surface antibody cocktail and incubate for another 15-20 minutes.
- Add 1-2 mL Cell Staining Buffer (Cat. No. 420201) or equivalent buffer containing BSA or serum. Centrifuge to pellet.
- Continue with normal fixation and permeabilization procedure. If planning to skip fixation and analyze cells live, complete an additional wash step to minimize any unnecessary background of the live cells.
- Notes: If the cell type in use cannot tolerate a protein-free environment, then titrate the Zombie NIR™ dye in the presence of the same amount of BSA/serum as will be present in the antibody staining procedure. A higher amount of Zombie NIR™ may be required since the BSA/serum will react with and bind up some proportion of the Zombie NIR™.
- Additional Product Notes
-
View more applications data for this product in our Scientific Poster Library.
-
Application References
(PubMed link indicates BioLegend citation) - Product Citations
-
Antigen Details
- Biology Area
- Apoptosis/Tumor Suppressors/Cell Death, Cell Biology, Neuroscience
- Gene ID
- NA
