- Clone
- 12F4 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- AAA, ABETA, ABPP, AD1, APPI, CTFgamma, CVAP, PN-II, PN2, Amyloid beta A4 protein, preA4, protease nexin-II, peptidase nexin-II, beta-amyloid peptide, alzheimer disease amyloid protein, cerebral vascular amyloid peptide, APP, Amyloid Precursor Protein
- Previously
-
Signet Catalog# 9142-02
Signet Catalog# 9142-05
Signet Catalog# 9142-10
Covance Catalog# SIG-39142
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
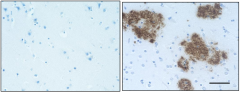
-
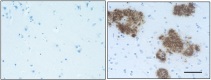
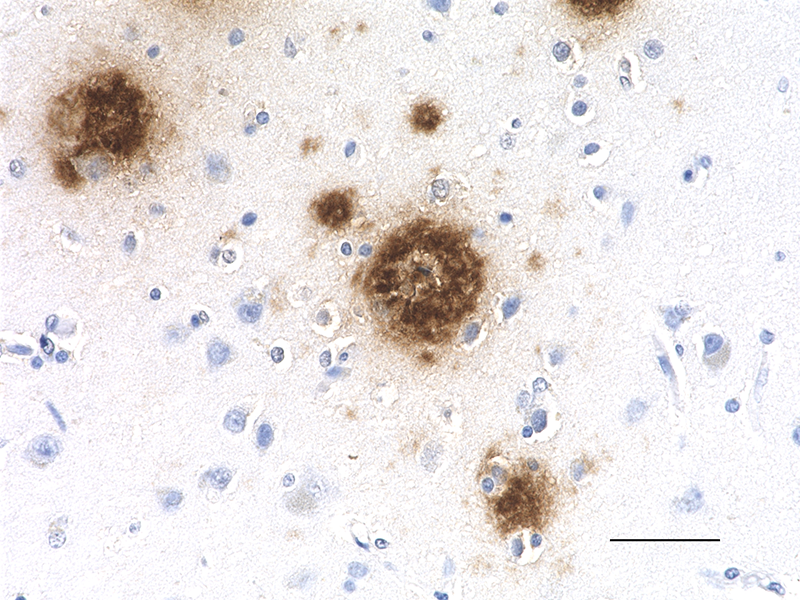
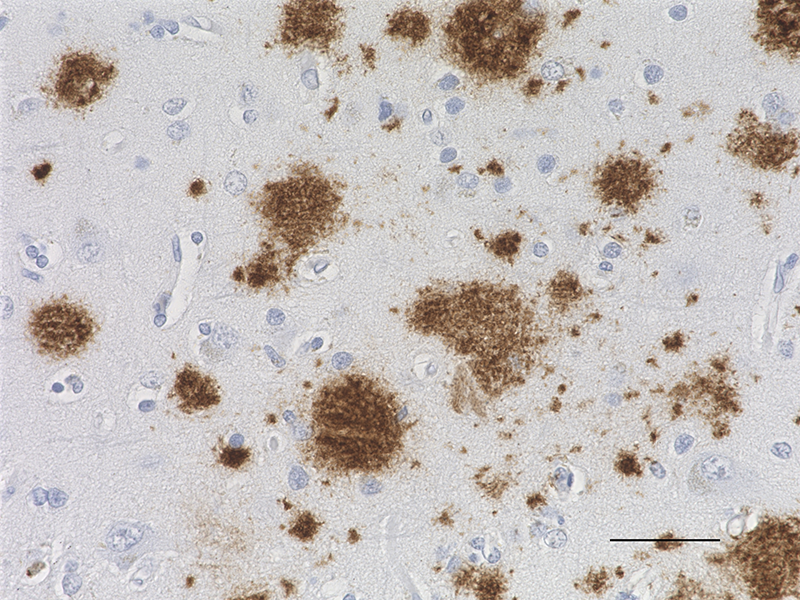
IHC staining of the purified anti-β-Amyloid, 1-42 antibody (12F4) on the formalin-fixed paraffin-embedded brain tissues from normal human (left panel) and patients with Alzheimer’s disease (left panel) and normal human brain tissue (right panel). Following antigen retrieval using 70% formic acid for 20 min, the tissue was incubated overnight with 1µg/mL of the primary antibody at 4°C. BioLegend´s Ultra-Streptavidin (USA) HRP kit (Multi-Species, DAB, Cat. No. 929901) was used for detection followed by hematoxylin and bluing solution counterstaining, according to the protocol provided. The image was captured with a 40X objective. Scale bar: 50 µm. -

Direct ELISA of the purified anti-β-amyloid 1-42 (clone 12F4) antibody binding to the plate-immobilized human and rodent Aβ1-42, human Aβ1-40 peptides, and recombinant human APP751 protein. ELISA was performed by coating the wells with 100 ng of peptide or recombinant protein. The wells were then incubated with the primary antibody at 37°C for 45 minutes, followed by incubation with the horseradish peroxidase labeled goat anti-mouse IgG secondary antibody. TMB (3, 3', 5, 5' tetramethylbenzidine, Cat. No. 421501) was used as the detection system. -
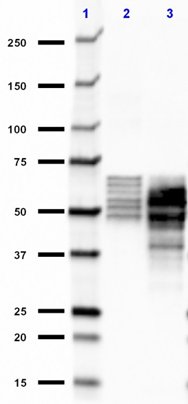
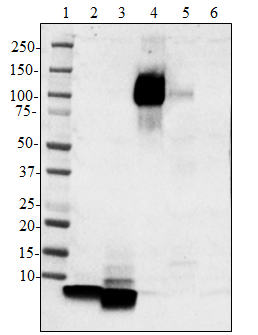
Western blot of the purified anti-β-amyloid, 1-42 antibody (clone 12F4). Lane 1: Molecular weight marker; Lane 2: 50 ng of the human Aβ1-40 peptide; Lane 3: 50 ng of the Aβ1-42 peptide. The blot was incubated with 1 µg/ml of the primary antibody overnight at 4oC, followed by incubation with the HRP-labeled goat anti-mouse IgG (cat: 405306). Enhanced chemiluminescence was used as the detection system.
Amyloid beta (Aβ or Abeta) denotes peptides of 36–43 amino acids in length that are crucially involved in Alzheimer's disease as the main component of the amyloid plaques found in the brains of Alzheimer patients. The peptides result from the amyloid precursor protein (APP), which is cut by certain enzymes to yield Aβ. Aβ molecules can aggregate to form oligomers (known as "seeds") which are believed to be able to induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The seeds or the resulting amyloid plaques are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.
Product DetailsProduct Details
- Verified Reactivity
- Human, Mouse, Rat
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- Phosphate-buffered solution (no preservatives or carrier proteins).
- Preparation
- The antibody was purified by affinity chromatography.
- Concentration
- 0.5 mg/ml
- Storage & Handling
- This antibody should be handled aseptically as it is free of preservatives such as Sodium Azide. Store this antibody undiluted between 2°C and 8°C. Please note the storage condition for this antibody has been changed from -20°C to between 2°C and 8°C. You can also check the vial label or CoA to find the proper storage conditions.
- Application
-
IHC-P - Qualty tested
Direct ELISA, WB - Verified
IHC-F, ELISA - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by formalin-fixed paraffin-embedded immunohistochemical staining. For immunohistochemistry, a concentration range of 0.2 - 5.0 µg/ml is suggested. For Direct ELISA, a concentration range of 0.015 - 1.5 µg/ml is suggested. For Western blotting, the suggested use of this reagent is 1.0 - 5.0 µg/ml. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
This antibody is effective in immunoblotting (WB), immunohistochemistry (IHC), and ELISA.
This antibody is reactive to the C-terminus of beta amyloid and is specific for the isoform ending at the 42nd amino acid.
Additional reported applications (for the relevant formats) includes: immunohistochemical staining of paraformaldehyde-fixed frozen sections6 and ELISA3,4,5. -
Application References
(PubMed link indicates BioLegend citation) -
- Helwig M, et al. 2013. J. Biol. Chem. 288:1114. (IHC-P)
- Takami M, et al.2009. J. Neurosci.; 29: 13042. (IHC-P)
- Cenini G, et al. 2012. Biochim Biophys Acta. 1822(2):130. (ELISA) PubMed
- Sundaram RK, et al. 2012. Int. J. Pept. Res. Ther. 18(2):99. (ELISA) PubMed
- Savage MJ, et al. 2014. J. Neurosci. 8:2884. (ELISA) PubMed
- Mastrangelo MA, Bowers WJ. 2008. BMC Neurosci. 9:81. (IHC-F) PubMed
- Product Citations
-
- RRID
-
AB_2564682 (BioLegend Cat. No. 805509)
AB_2564682 (BioLegend Cat. No. 805501)
AB_2564682 (BioLegend Cat. No. 805502)
AB_2564682 (BioLegend Cat. No. 805503)
Antigen Details
- Structure
- Amyloid precursor protein is a 770 amino acid protein with a molecular mass of ~100 kD. According to the UniProtKB database, APP (ID# P05067) has 11 isoforms (34 to ~90 kD) and the 770 form has been designated as the canonical form. Isoform APP695 is the predominant form expressed in neuronal tissue. Isoforms APP751 and APP770 are widely expressed in non-neuronal cells. Isoform APP751 is the most abundant form in T-lymphocytes. Aβ denotes peptides of 36-43 amino acids generated from cleavage of APP by secretases. Aβ has an apparent molecular mass of about 4 kD.
- Distribution
-
Tissue sources: Primarily nervous system, but also adipose tissue, intestine, muscle.
Distribution: cytosol, endosomes, nucleus, plasma membrane, extracellular, and golgi apparatus. - Function
- The normal function of Aβ is not well understood. Several potential physiological roles have been proposed, including: activation of kinase enzymes; protection against oxidative stress; regulation of cholesterol transport; transcription factor, and as an anti-microbial agent.
- Biology Area
- Cell Biology, Neurodegeneration, Neuroscience, Protein Misfolding and Aggregation
- Molecular Family
- APP/β-Amyloid
- Antigen References
-
- Kumar A, et al. 2015. Pharmacol. Rep. 67(2):195.
- Sadigh-Eteghad S, et al. 2015. Med. Princ. Pract. 24(1):1
- Hampel H, et al. 2015. Expert Rev. Neurother. 15(1):83.
- Puig KL, et al. 2012. Exp. Gerontol. 48(7): 608.
- Selkoe DJ, et al. 2016. EMBO Mol. Med. 8(6):595.
- Walsh DM, et al. 2007. J. Neurochem. 101(5):1172.
- Gene ID
- 351 View all products for this Gene ID
- UniProt
- View information about beta-Amyloid 1-42 on UniProt.org
Related Pages & Pathways
Pages
Other Formats
View All β-Amyloid, 1-42 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Biotin anti-β-Amyloid, 1-42 | 12F4 | IHC-P,Direct ELISA |
| HRP anti-β-Amyloid, 1-42 | 12F4 | IHC-P,WB |
| Purified anti-β-Amyloid, 1-42 | 12F4 | IHC-P,Direct ELISA,WB,IHC-F |
Customers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Biotin anti-β-Amyloid, 1-42
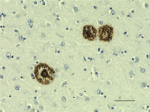
IHC staining of Biotin anti-β-Amyloid, 1-42 antibody (clone ... 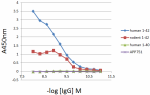
Direct ELISA of Biotin anti-β-Amyloid, 1-42 antibody (clone ... -
HRP anti-β-Amyloid, 1-42
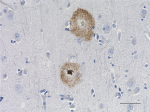
IHC staining of HRP anti-β-Amyloid, 1-42 antibody (clone 12F... 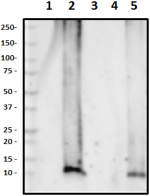
Western blot of HRP anti-β-Amyloid, 1-42 antibody (clone 12F... -
Purified anti-β-Amyloid, 1-42
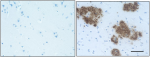
IHC staining of the purified anti-β-Amyloid, 1-42 antibody (... 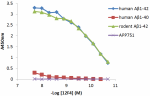
Direct ELISA of the purified anti-β-amyloid 1-42 (clone 12F4... 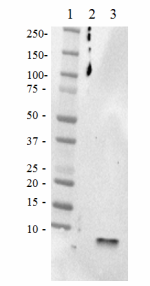
Western blot of the purified anti-β-amyloid, 1-42 antibody (...

 Login / Register
Login / Register 












Follow Us