- Regulatory Status
- RUO
- Other Names
- MMP9, CLG4B, GELB, MANDP2, MMP-9, 92 kDa type IV collagenase, 92 kDa gelatinase, gelatinase B, matrix metallopeptidase 9
- Ave. Rating
- Submit a Review
- Product Citations
- publications
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 763401 | 4 pack | £65 | ||||
MMP-9 is a member of the matrix metalloprotease family of proteins (MMPs). Members of this family are structurally related, zinc-containing enzymes that degrade the extracellular matrix (ECM) and connective tissue proteins. Proteolytic activities of MMPs play an important role in vascular remodeling, cellular migration, and the processing of ECM proteins and adhesion molecules. MMP-9 consists of a prodomain, catalytic domain, hinge region, and a hemopexin domain, and is secreted as an inactive zymogen that becomes activated extracellularly. The most relevant natural activators of proMMP-9 are unknown. MMP-9 activation may be mediated by removal of the prodomain by serine proteases or other MMPs, or it may be a direct response to oxidative stress that disrupts the cysteine switch. MMP-9 is capable of processing cytokines and chemokines. For example, it is reported to release the biologically active form of vascular endothelial growth factor (VEGF), indicating that MMP-9 may play a crucial role in the formation of new blood vessels. MMP-9 participates in the migration of immune cells as demonstrated by MMP-9 knockout mice after antigen challenge. Increased MMP-9 expression and activity has been observed to be related to various cancers, as it may contribute to the release of metastatic cells from the bulk tumor as well as their entrance at the site of metastasis by degrading proteins in ECM and basement membrane.
Product DetailsProduct Details
- Source
- Human MMP-9, amino acids (Ala20-Asp707) (Accession# NP_004985.2), is expressed with a C-terminal 8His tag and a linker sequence in the 293E cell line.
- Molecular Mass
- This 722 amino acid recombinant protein has a predicted molecular mass of approximately 80 kD. The protein migrates at about 92 kD in DTT-reducing conditions and about 92 kD in non-reducing condition by SDS-PAGE. The predicted N-terminal amino acid is Ala.
- Purity
- > 95%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- Lyophilized in sterile-filtered PBS, pH 7.2, containing 1% BSA, 0.09% sodium azide, and protease inhibitors.
- Concentration
- Lot-specific (to obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.)
- Storage & Handling
- Unopened vials can be stored between 2°C and 8°C until the expiration date. Prior to use, reconstitute the lyophilized powder with 0.2 mL of PBS containing a carrier protein (e.g., 1% BSA, protease free), pH7.4. Re-cap vial, vortex. Allow the reconstituted standard to sit at room temperature for 15 minutes, vortex again to mix completely. The reconstituted standard stock solution can be aliquoted into polypropylene vials and stored at -70°C for up to one month. Do not re-use diluted standards. Avoid repeated freeze/thaw cycles.
- Application
-
ELISA - Quality tested
- Recommended Usage
-
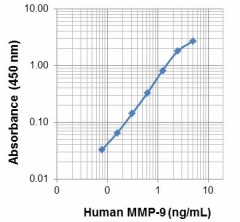
Each lot of this protein is quality control tested by ELISA assay. For use as an ELISA standard, a standard curve comprised of doubling dilutions from 0.078 ng/ml to 5 ng/ml is suggested. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
This MMP-9 protein is useful as a standard for a human MMP-9 sandwich ELISA, using unlabeled M2101G05 antibody (Cat. No. 681802) for capture and biotinylated M2108F07 antibody (Cat. No. 532101) for detection.
Note: For testing human MMP-9 in serum or plasma, BioLegend's LEGEND MAX™ Kit (Cat. No. 444907) is specially developed and recommended.
Antigen Details
- Structure
- Monomer
- Distribution
-
MMP-9 is expressed by alveolar macrophages, granulocytes, fibroblasts, and fibrosarcoma cells
- Function
- Degradation of ECM and connective tissue proteins. Angiogenesis, tissue remodeling, and cancer metastasis. Regulates chemokine activity. Inhibited by TIMPs and α2-macroglobulin (inhibitor of MMPs in bodily fluids).
- Interaction
- ECM proteins
- Ligand/Receptor
- TIMPs
- Biology Area
- Angiogenesis, Cell Adhesion, Cell Biology, Neuroinflammation, Neuroscience
- Molecular Family
- Enzymes and Regulators
- Antigen References
-
- Chakrabarti S, Patel KD. 2005. Exp. Lung Res. 31:599.
- Klein T, Bischoff R. 2011. Amino Acids 41:271.
- Raffetto JD, Khalil RA. 2008. Biochem. Pharmacol. 75:346.
- Bourboulia D, Stetler-Stevenson WG. 2010. Semin. Cancer Biol. 20:161.
- Vilen ST, et al. 2013. Scientific World Journal. 920595.
- Gene ID
- 4318 View all products for this Gene ID
- UniProt
- View information about MMP-9 on UniProt.org
 Login / Register
Login / Register 
















Follow Us