- Regulatory Status
- RUO
- Other Names
- Fixable Dye (live/dead), Fixable Viability Dye,Dead Cell Stain Kit, Dead Cell Staining Kit
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

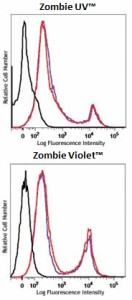
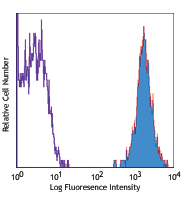
One day old C57BL/6 mouse splenocytes were stained with Zombie dyes as indicated and analyzed before fixation (blue/purple) or after fixation and permeabilization (red). Cells alone, without Zombie staining, are indicated in black. -

One day old C57BL/6 mouse splenocytes were stained with Zombie dyes as indicated and analyzed before fixation (blue/purple) or after fixation and permeabilization (red). Cells alone, without Zombie staining, are indicated in black. -

One day old C57BL/6 mouse splenocytes were stained with Zombie dyes as indicated and analyzed before fixation (blue/purple) or after fixation and permeabilization (red). Cells alone, without Zombie staining, are indicated in black.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 423117 | 1 kit | £241 | ||||
The Zombie dyes in this kit (provided at 100 tests each) are the UV, NIR, Violet, Aqua and Yellow variants.
The Zombie live/dead fixable dyes are amine-reactive fluorescent dyes that are impermeant to live cells but permeant to cells with compromised membranes. They irreversibly conjugate to primary amine-containing proteins. Since there is a greater abundance of proteins inside the cell versus the cell surface, dead cells with a compromised membrane stain brighter than live cells with an intact membrane. Thus, they can be used to assess live vs. dead status of mammalian cells. However, titration of the reagent is required to ensure that live cells have minimal to no staining due to this dye on the cell type of interest. It is also important to consider that the accuracy of the live/dead assessment is dependent on the uniformity in size of each cell since larger cells will stain brighter than smaller cells, possibly confusing large cells as dead cells. Zombie live/dead fixable probes are useful for incorporation into multicolor panels, particularly in applications where cells will be fixed and permeabilized prior to analysis.
To find the spectra of each of the Zombie dyes offered in this kit, please find them here: https://www.biolegend.com/spectraanalyzer
Product Details
- Preparation
- Zombie Fixable Viability Kit is composed of 5 vials of 100 tests of each lyophilized Zombie dye and anhydrous DMSO. For reconstitution, bring the kit to room temperature only when ready to use the reagent; add 100 µl of DMSO to each vial of Zombie dye until fully dissolved.
- Storage & Handling
- Store kit at -20°C upon receipt. Do not open vials until needed. Once the DMSO is added to the Zombie dye, use immediately, or store at -20°C in a dry place and protected from light, preferably in a desiccator or in a container with desiccant for no more than one month. Please contact technical support for lot specific CoA and expiration date inquiries of this product.
- Application
-
FC - Quality tested
ICFC, ICC - Verified - Recommended Usage
-
Each lot of this reagent is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is 1:100-1:1000 dilution per 1-10 million cells in 100 µL volume. For immunocytochemistry, the suggested dilution is 1:1000. It is recommended that the reagent be titrated for optimal performance for each application, as optimal dosage varies with cell type.
- Application Notes
-
Standard Cell Staining Protocol:
- Prior to reconstitution, spin down the vial of lyophilized reagent in a microcentrofuge to ensure the reagent is at the bottom of the vial.
- For reconstitution, pre-warm the kit to room temperature; add 100 µL of DMSO to one vial of Zombie dye and mix until fully dissolved
- Wash cells with PBS buffer (no Tris buffer and protein free).
- Dilute Zombie dye at 1:100-1000 in PBS. Resuspend 1-10 x 106 cells in diluted 100 µL Zombie solution. To minimize background staining of live cells, titrate the amount of dye and/or number of cells per 100 µL for optimal performance. Different cell types can have a wide degree of variability in staining based on cell size and degree of cell death.
- Note: Don’t use Tris buffer as a diluent and be sure that the PBS does not contain any other protein like BSA or FBS.
- Note: The amount of dye used can also influence the ability to detect apoptotic as well as live and dead cells.
- Incubate the cells at room temperature (or 4°C), in the dark, for 15-30 minutes.
- Wash one time with 2 ml BioLegend’s Cell Staining Buffer (Cat. No. 420201) or equivalent buffer containing serum or BSA.
- Continue performing antibody staining procedure as desired.
- Cells can be fixed with paraformaldehyde or methanol prior to permeabilization or can be analyzed without fixation.
No-wash Sequential Staining Protocol:
- Wash cells with PBS buffer (no Tris buffer and protein free).
- For reconstitution, pre-warm the kit to room temperature; add 100 µL of DMSO to one vial of Zombie dye and mix until fully dissolved
- Determine the total µL volume of antibody cocktail previously titrated and optimized for the assay that will be added to each vial/well of cells based on a final volume of 100 µL. Subtract that antibody volume from the 100 µL total staining volume intended for the assay. In the remaining volume, dilute Zombie dye at 1:100-1000 in PBS as determined by prior optimization at that volume. For example, if you are adding 20 µL of antibody cocktail for a 100 µl total staining volume, use 80 µL of Zombie solution. Resuspend 1-10 x 106 cells in the appropriate volume of Zombie solution. Different cell types can have a wide degree of variability in staining based on cell size and degree of cell death.
- Note: Don’t use Tris buffer as a diluent and be sure that the PBS does not contain any other protein like BSA or FBS.
- Note: The amount of dye used can also influence the ability to detect apoptotic as well as live and dead cells.
- Incubate for 10-15 minutes at RT (or 4°C), protected from light. Without washing the cells, add the cell surface antibody cocktail and incubate for another 15-20 minutes.
- Add 1-2 mL Cell Staining Buffer (Cat. No. 420201) or equivalent buffer containing BSA or serum. Centrifuge to pellet.
- Continue with normal fixation and permeabilization procedure. If planning to skip fixation and analyze cells live, complete an additional wash step to minimize any unnecessary background of the live cells.
- Notes: If the cell type in use cannot tolerate a protein-free environment, then titrate the Zombie dye in the presence of the same amount of BSA/serum as will be present in the antibody staining procedure. A higher amount of Zombie dye may be required since the BSA/serum will react with and bind up some proportion of the Zombie dye.
- Product Citations
-
Antigen Details
- Biology Area
- Apoptosis/Tumor Suppressors/Cell Death, Cell Biology, Neuroscience
- Gene ID
- NA
Related Pages & Pathways
Pages
Related FAQs
- I am concerned about the spillover I am observing from the Zombie dye into its neighboring channels.
- Rule of thumb with Zombie dyes is to titrate them down as much as possible to fit your application. This should potentially help with spillover. Secondly, Zombie positive events represent dead cells and are typically gated out from analysis.
- How does the performance of your Zombie dye compare with competitors?
-
Zombie dyes have been tested against other leading competitors' fixable viability kits and given comparable results. We also highly recommend that you titrate down the amount of each dye used in order to best match the negative signals of your unstained sample and MFI- (mean fluorescence intensity) stained samples.
- Can I use methanol/ethanol for fixation after using a Zombie dye?
-
Yes, most fixation reagents are fine to be used with Zombie dyes. However, it should be noted that Zombie dyes can still be sensitive to reactive oxygen species. Light exposure or reagents with hydrogen peroxide can lead to free radical formation, affecting fluorescence.
- Can Zombie be used to determine bacteria, yeast viability?
- We have not tested in house bacterial or yeast viability using Zombie dyes. It is not clear whether the difference between surface and intracellular signals will be significantly different in case of non mammalian cells.
- Can I use Zombie with cells suspension containing serum?
- Serum is full of proteins which will sequester the dye and thereby reducing its effective concentration. The basic rule of thumb with zombie is to titrate it based on your specific condition. Titration also helps reduce the background and spillover into other channels.
- Can I use Zombie dyes for microscopy?
-
Zombie dyes tested in-house for microscopy applications will display data on the product technical datasheet. It should be noted that Zombie dyes may not work for dead cell discrimination in every microscopy application. Important considerations that may impact analysis are determining the signal level that constitutes a dead cell and identifying the proper plane to observe the dead cells.
- Why can't I fix my cells prior to using Zombie dyes?
-
The fixation process can contort and alter the membrane of cells, effectively rendering them dead. Since the ability of the Zombie dyes to stain dead cells is correlated with cell permeability, your results may no longer be a valid representation of dead versus live cells.
- Can I use Zombie dyes to detect apoptotic cells?
-
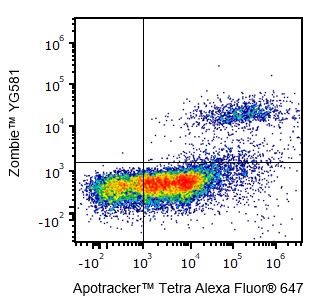
Yes, Zombie dyes can be used with apoptosis markers, such as Annexin V or Apotracker™ (shown below), to discriminate live, apoptotic, and dead cells.
One day-old C57BL/6 mouse thymocytes were stained with Apotracker™ Tetra Alexa Fluor® 647 and Zombie™ YG581. Zombie-dim/Apotracker™-positive cells are apoptotic, while double-positive cells are dead. Live cells are negative for both markers.
- How should I store Zombie dyes?
-
Store the Zombie dye kit at -20°C upon receipt. Do not open vials until needed. Once DMSO is added, use immediately or store at -20°C in a dry place and protected from light, preferably in a desiccator or in a container with desiccant for no more than one month.
 Login / Register
Login / Register 














Follow Us