BioLegend Cell-Vive™ GMP Ultra-LEAF™ antibodies are manufactured and tested in accordance with USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016.
Find complete GMP manufacturing quality and regulatory statements here.
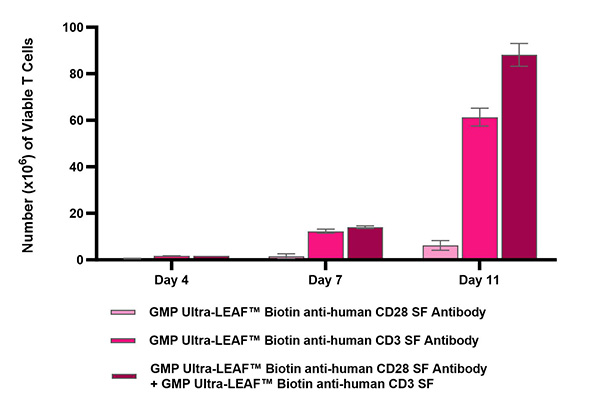
View all GMP Ultra-LEAF™ antibodies.
Product specifications and manufacturing guidelines include:
 Login / Register
Login / Register 











Follow Us