- Regulatory Status
- RUO
- Other Names
- Transforming growth factor-beta 2 (TGF-b2)
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
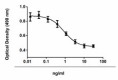
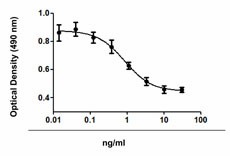
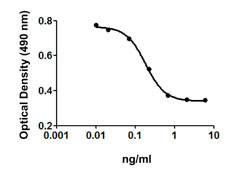
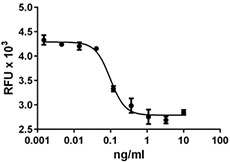
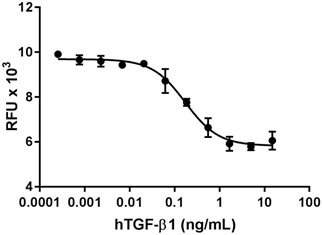
Inhibition of IL-4 induce HT-2 cell proliferation by TGF-β2.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 583301 | 5 µg | 317€ | ||||
Human TGF-β2 consists of two disulfide-linked, identical subunits, and displays 71.4% sequence homology with TGF-β1. TGF-β2 is synthesized in cells as a 442 amino acid. Furin cleaves the protein, yielding an N-terminal cleavage product which corresponds to latency-associated peptide (LAP), and the 25 kD C-terminal portion of the precursor constitutes the mature TGF-β2. TGF-β activators can release TGF-β from LAP. These activators include proteases that degrade LAP, thrombospondin-1, reactive oxygen species, and integrins avb6 and avb8. TGF-β2 is the predominant form of TGF-β in ocular tissues, and elevated levels of TGF-β2 have been found in the aqueous humor of patients with primary open-angle glaucoma (POAG), a major cause of blindness worldwide. There is an accumulation of extracellular matrix (ECM) in the trabecular meshwork (TM) of glaucoma patients, and TGF-β2 seems to be responsible for this ECM increase. It has been identified that bone morphogenetic protein-4 and 7 (BMP4, BMP7) are potent antagonists of the fibrogenic effects of TGF-β2 on human TM cells. Smad7 seems to participate in the antagonistic effect of BMP7 on TGF-β2 signaling.
Product DetailsProduct Details
- Source
- Human TGF-β2, amino acids Ala303-Ser414 (Accession# NM_001135599.2) was expressed in CHO cells.
- Molecular Mass
- The 112 amino acid recombinant protein has a predicted molecular mass of approximately 12.7 kD. The DTT-reduced protein migrates at approximately 14 kD and non-reduced protein migrates at 28-30 kD by SDS-PAGE. The N-terminal amino acid is Alanine.
- Purity
- >98%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in 20% Acetonitrile, 0.1% TFA (Trifluoroacetic acid).
- Endotoxin Level
- Less than 0.01 ng per µg cytokine as determined by the LAL method.
- Concentration
- 5 µg size is bottled at 100 µg/mL.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- TGF-β2 inhibits the proliferation of mouse HT-2 cells induced by IL-4. ED50 = 1 - 4 ng/ml, corresponding to a specific activity of 0.25 - 1.0 x 106 units/mg.
- Application
-
Bioassay
- Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
- Product Citations
-
Antigen Details
- Structure
- Homodimer
- Distribution
-
Mature phagocytic cells, CD34+ human bone marrow cells, and neutrophils. TGF-β2 is secreted by numerous cells.
- Function
- TGF-β2 stimulates proliferation of hematopoietic stem and progenitor cells, induces the expression of ECM proteins, and induces VEGF in retinal pigment epithelium and mucin in human bronchial epithelial cells. Also, TGF-β2 induces differentiation, apoptosis, and angiogenesis. TGF-β2 expression in human bronchial epithelial cells is induced by IL-4 and IL-13 and decreased by IFNγ.
- Interaction
- Bronchial epithelium, mesangial cells, fibroblasts, human retinal pigment epithelium, optic nerve head astrocytes, and lamina cribrosa cells. The majority of mammalian cells express TGF-β receptors.
- Ligand/Receptor
- Type I (ThRI), and type II (ThRII) receptors.
- Cell Type
- Embryonic Stem Cells, Mesenchymal Stem Cells, Neural Stem Cells
- Biology Area
- Cell Biology, Signal Transduction, Stem Cells
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
1. Wen FQ, et al. 2002. Am. J. Respir. Cell. Mol. Biol. 26:484.
2. Chu HW, et al. 2004. Am. J. Pathol. 165:1097.
3. Park SH, 2005. J. Biochem. Mol. Biol. 38:9.
4. Bian ZM, et al. 2007. Exp. Eye Res. 84:812.
5. Suzuki K, et al. 2007. Cancer Res. 67:3673.
6. Fuchshofer R, et al. 2009. Exp. Eye Res. 88:1020.
7. Shah CA, et al. 2011. J. Biol. Chem. 286:3161.
8. Zode GS, et al. 2011. Molecular Vision 17:1745. - Gene ID
- 7042 View all products for this Gene ID
- UniProt
- View information about TGF-beta2 on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 









Follow Us