Deciphering B Cell Cancers With a Rituximab Biosimilar
Cancer is one of the largest health problems worldwide, accounting for one in every six deaths1 (Figure 1). By 2040, the global cancer burden is estimated to increase to 27.5 million cases and 16.3 million deaths factoring in the growth and aging of the population2.
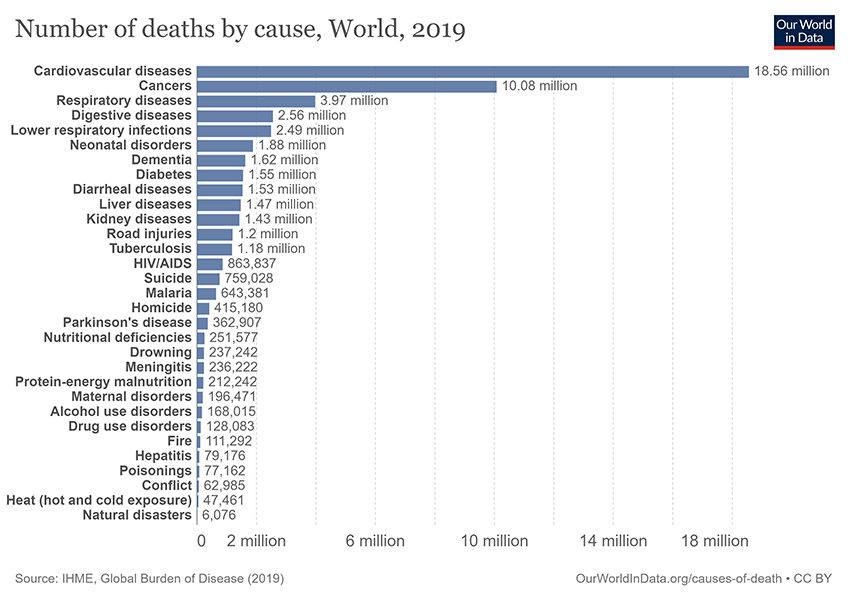
Figure 1. Number of deaths attributed to different causes worldwide from 1990 to 20181.
The history of cancer research and treatment has spanned 250 years3, with immunotherapies being among the most current approaches. Immunotherapies now consist of a number of different approaches, such as monoclonal antibodies (mAbs), vaccines, checkpoint inhibitors, cytokines, and CAR cell therapy. In this blog, we focus on advancements concerning research and treatment of B cell cancers.
As B cells develop, they begin expressing the CD20 marker, which is reported to play a role in the proliferation, activation, and cell cycle of human B cells. CD20 is also expressed on malignant B cells, with increased expression found in patients with B cell lymphoma and leukemia, such as Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia4. High levels of CD20 markers on the surface of B cells, but not on other normal cells, have made them attractive targets for antibody-based therapy.
Rituximab is an antibody therapy that targets and attaches to the CD20 surface markers on either normal B cells or B cell cancers. Upon binding to CD20, Rituximab triggers the destruction of B cells by several characterized mechanisms5, including complement-mediated cytotoxicity (CMC), antibody-dependent cellular cytotoxicity (ADCC), and stimulation of the apoptotic pathway (Figure 2).
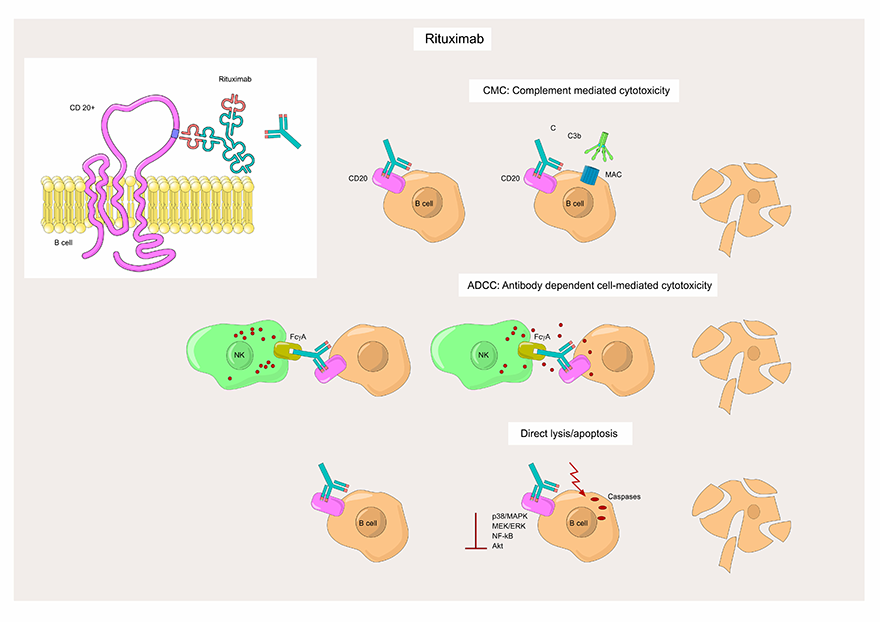
Figure 2. Structure and mechanism of action of Rituximab anti-CD20 monoclonal antibody.
Thus, Rituximab exerts its effects by targeted depletion of B cells, so it is used as therapy for oncological and autoimmune diseases characterized by excessive, overactive, or dysfunctional B cells5.
Anti-CD20 therapies are now vastly practiced and highly effective at treating cancer and immune-related disorders. But, they are among the most expensive treatments, straining healthcare budgets and putting biologics out of reach for many patients6. These barriers have spurred development of therapeutic biosimilars, which are highly similar to already approved, FDA-licensed biologics but at lower costs7.
Use of therapeutic biologics, however, can also be cost-prohibitive for those researching cancers and potential therapeutics. This is where research-grade biosimilars can bridge the need and keep research costs down. At BioLegend, we are driven to providing cancer researchers the highest quality tools. Based on published sequence for the Rituximab therapeutic antibody, our Ultra-LEAF™ purified anti-human CD20 recombinant antibody (clone QA20A02) is a Rituximab biosimilar, designed for biosimilar research and development. Similar to the original therapeutic Rituximab mAb, our research-use-only (RUO) biosimilar is made using the Chinese hamster ovary (CHO) expression system. Ideal for laboratories in academia, pharmaceutical, and biotechnology industries investigating new drugs, our biosimilar addresses market needs for products that mimic approved biological drugs for research use.
To evaluate the biological activity of our Rituximab RUO biosimilar, we employed a variety of in vitro functional assessments. First, our representative data indicate our biosimilar (Figure 3B) binds to CD20-expressing cells at a comparative quality to a competitor (Figure 3C).
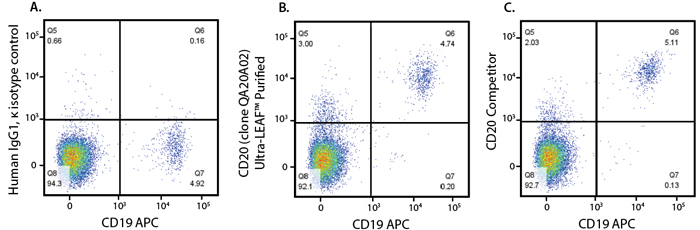
Figure 3. Human peripheral blood lymphocytes were stained with Ultra-LEAF human IgG1 isotype control (A), Ultra-LEAF Purified anti-human CD20 (Rituximab Biosimilar, RUO) Recombinant Antibody (clone QA20A02) (B), or anti-human CD20 (Rituximab Biosimilar) from another supplier (C), followed by PE anti-human IgG Fc. Cells were co-stained with anti-human CD19.
Second, our representative functional assay shows induction of complement-dependent cytotoxicity (CDC) by our biosimilar is also comparable to that of a competitor (Figure 4).
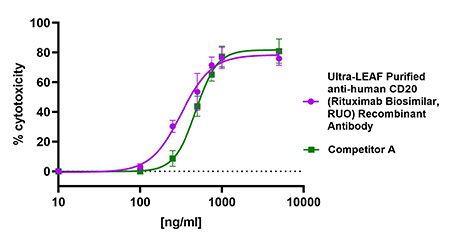
Figure 4. Ultra-LEAF Purified anti-human CD20 (Rituximab Biosimilar, RUO) Recombinant Antibody binds to human CD20 on the surface of Daudi cells and activates complement-dependent cytotoxicity (CDC) in a dose-dependent manner similar to another Rituximab biosimilar from a competitor (green). This antibody has an IC50 range of 200-500 ng/mL.
Lastly, glycosylation is a common post-translational modification for IgG antibodies produced by mammalian cells, such as the frequently employed CHO cell. Changes in the glycosylation pattern of proteins can impact their stability, efficacy, and function8. In the representative chromatograms shown, similar glycan profiles demonstrate lot-to-lot consistency of our RUO biosimilar.
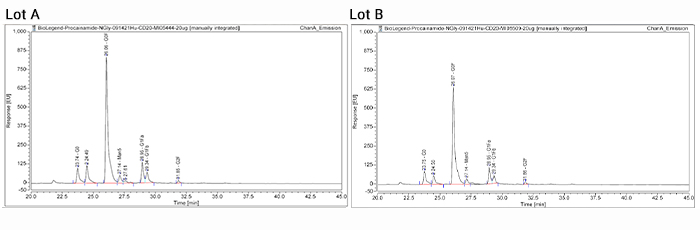
In summary, our Ultra-LEAF™ purified anti-human CD20 recombinant antibody (clone QA20A02) is a RUO biosimilar to the therapeutic mAb Rituximab. Our biosimilar features the variable region of Rituximab, making it ideal to further research CD20 mechanism of action in the lab. Insights gained from such studies could then be used to further clinical research and other applications. Our RUO biosimilar is purified by affinity chromatography and validated by flow cytometry. In addition, we provide our biosimilar in our exclusive Ultra-LEAF functional antibody formulation, which guarantees the antibodies have ultra-low levels of endotoxin (< 0.01 EU/μg) and are azide-free. As our in vitro functional assessments show, our RUO biosimilar targets CD20 on B cells, triggers cell-mediated cytotoxicity, and is of exceptional quality and consistency. This makes it ideal for researchers studying B cell cancers and for use in research and development of potential anti-tumor therapeutics.
References
- Roser, Max. "Cancer." Our World in Data, 3 July 2015, ourworldindata.org/cancer.
- Global Cancer Facts & Figures. American Cancer Society. www.cancer.org/research/cancer-facts-statistics/global.html.
- “Milestones in Cancer Research and Discovery.” National Cancer Institute, 31 Aug. 2020, www.cancer.gov/research/progress/250-years-milestones
- Payandeh, Zahra et al. “The applications of anti-CD20 antibodies to treat various B cells disorders.” Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie vol. 109 (2019): 2415-2426. doi:10.1016/j.biopha.2018.11.121. PubMed.
- Cobb, Patrick et al. “A review of the totality of evidence in the development of ABP 798, a rituximab biosimilar.” Immunotherapy vol. 14,9 (2022): 727-740. doi:10.2217/imt-2022-0024. PubMed.
- Cheesman, Simon. “Introduction of Biosimilar Rituximab: A Hospital Perspective.” HemaSphere, vol. 5, no. 1, Ovid Technologies (Wolters Kluwer Health), Dec. 2020, p. e515. https://doi.org/10.1097/hs9.0000000000000515. PubMed.
- What Are Biosimilar Drugs? | Biosimilar Drugs for Cancer Treatment. www.cancer.org/treatment/treatments-and-side-effects/treatment-types/biosimilar-drugs/what-are-biosimilars.html.
- Lee, Kyoung Hoon et al. “Analytical similarity assessment of rituximab biosimilar CT-P10 to reference medicinal product.” mAbs vol. 10,3 (2018): 380-396. doi:10.1080/19420862.2018.1433976. PubMed.
 Login/Register
Login/Register 






Follow Us