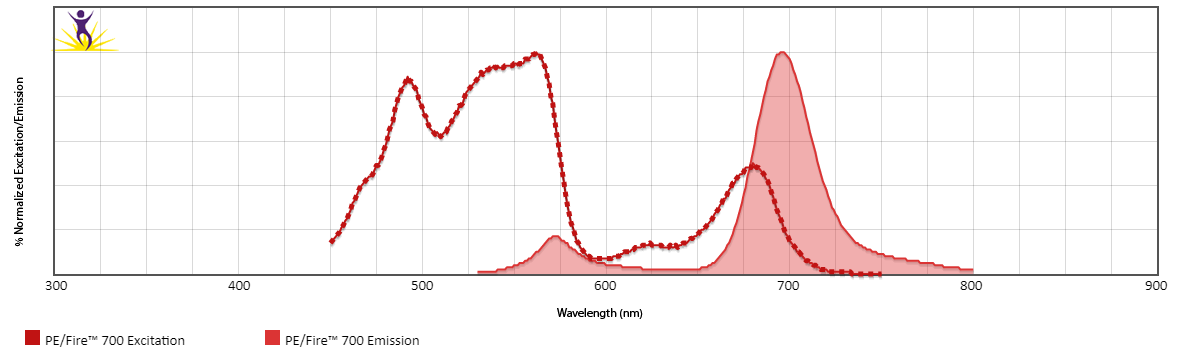
Excitation and Emission Spectra of PE/Fire™ 700
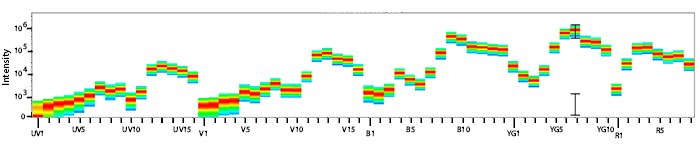
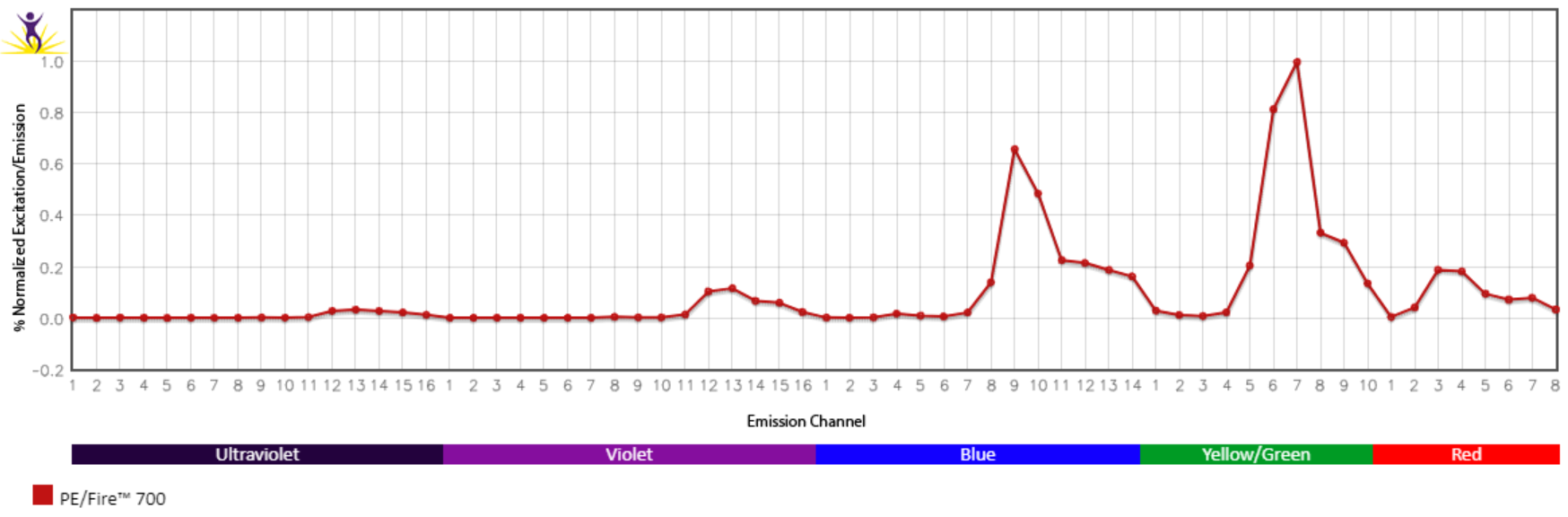
Emission spectra (top) and normalized emission spectra (middle) of PE/Fire™ 700 as run on a 5-laser Cytek® Aurora Spectral Cytometer. To compare PE/Fire™ 700 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
Normalized excitation and emission spectra (bottom) of PE/Fire™ 700 obtained from a spectrophotometer. To compare PE/Fire™ 700 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
Spectral Spillover of PE/Fire™ 700

Spillover impact of PE/Fire™ 700 into detection channels of a 5-laser Cytek® Aurora Spectral Cytometer.
Multicolor Compatibility of PE/Fire™ 700
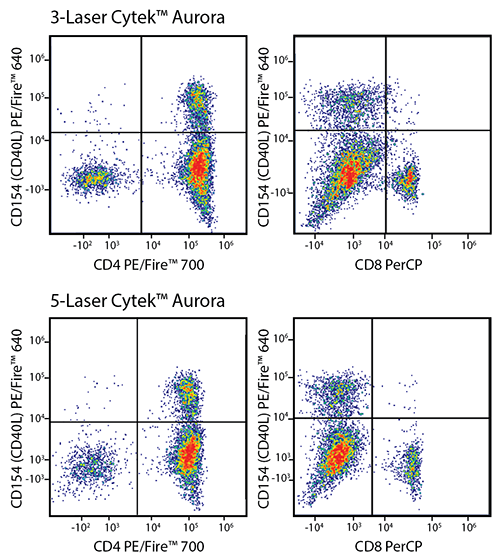
Multicolor Panel:
|
Marker
|
Clone
|
Fluorophore
|
|
CD4
|
SK3
|
PE/Fire™ 700
|
|
CD8
|
SK1
|
PerCP
|
|
CD19
|
HIB19
|
PE/Cyanine5
|
|
CD56
|
5.1H11
|
PE/Dazzle™ 594
|
|
CD154
|
24-31
|
PE/Fire™ 640
|
RBC-lysed and washed human blood was stained with a multicolor panel with an intentionally high degree of spectral complexity and overlap (left). Cells were analyzed on a 3-laser (top panels) or 5-laser Cytek® Aurora (bottom panels). Optimal test size concentrations were used for each antibody. Cells were gated on CD19-/CD56- events, and CD154 (CD40L) PE/Fire™ 640 is displayed against CD4 PE/Fire™ 700 or CD8 PerCP. This panel is able to be unmixed on both a 3-laser and a 5-laser Aurora. However, both the intensity of the PerCP signal and the spreading error of PE/Fire™ 640 is less substantial using a system that has both a 488 nm blue laser and a 561 nm yellow/green laser (Bottom panels). Spreading error only poses a problem when the brightness of the two fluors being plotted is not of sufficient intensity to resolve the two populations.
PE/Fire™ 700 Unmixing from PE/Cyanine5.5
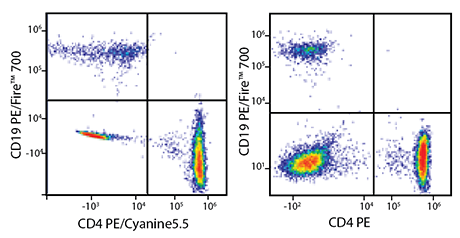
RBC-lysed and washed human blood was co-stained with anti-CD19 PE/Fire™ 700 and either anti-CD4 PE/Cyanine5.5 (left) or a non-overlapping control fluor – in this case, anti-CD4 PE (right). Cells were acquired on a 5-laser Cytek® Aurora and gated on lymphocytes. When compared to the non-overlapping control (right), cells co-stained with PE/Fire™ 700 and PE/Cyanine5.5 (left) display a similar pattern of percent positive populations. This shows that it is possible to unmix PE/Fire™ 700 from PE/Cyanine5.5, but panel design should be carefully considered.
PE/Fire™ 700 and PE/Cyanine5.5 Brightness Comparison
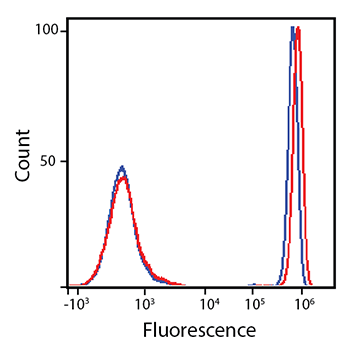
RBC-lysed and washed human blood was stained with anti-CD4 PE/Fire™ 700 (red) or anti-CD4 PE/Cyanine5.5 (blue).
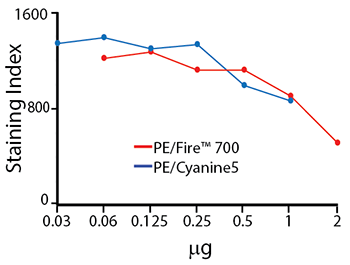
Titration Curve for PE/Fire™ 700
Titration of anti-CD4 PE/Fire™ 700 and anti-CD4 PE/Cyanine5 staining on RBC-lysed and washed human blood. Titrations were performed within the optimal range for each individual fluorophore. These two fluorophores are similar in brightness but both also exhibit significant spillover into neighboring emission channels. The staining index tends to decrease with greater concentrations of antibody used – this is due to an increase in non-specific binding.
PE/Fire™ 700 Stability
Photostability
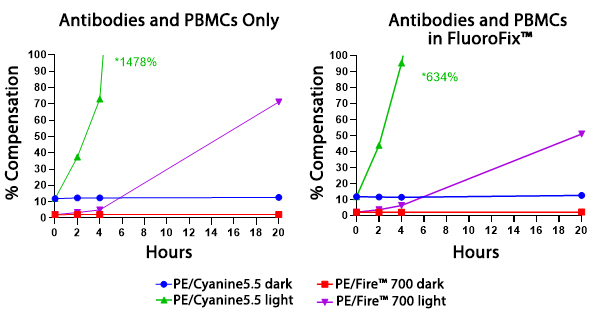
Two conditions of anti-CD4 PE/Fire™ 700 and anti-CD4 PE/Cyanine5.5 photostability were tested: (left) antibodies formulated at the optimal test concentration were left under fluorescent lighting or protected from light, then used to stain human PBMCs and (right) antibodies were used to stain human PBMCs, which were then fixed and stored in FluoroFix™ either under fluorescent lighting or protected from light. The long-term stability of antibody conjugates can be predicted by its brightness and, in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel.
Stability in Fixatives
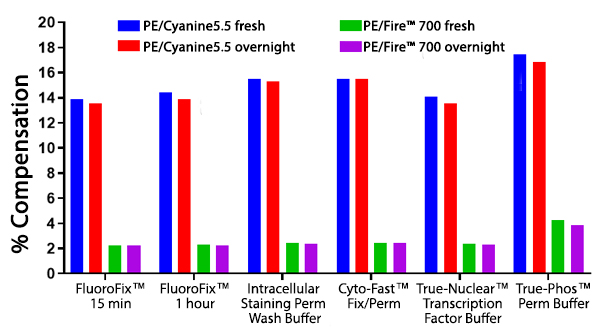
RBC-lysed and washed human blood cells were stained with anti-CD4 PE/Fire™ 700 or anti-CD4 PE/Cyanine5.5 and fixed according to the recommended protocols for each fixative. Cells were analyzed directly after fixation and washing (fresh), or analyzed after being stored overnight in Cyto-Last™ Buffer. PE/Fire™ 700 is resistant to paraformaldehyde fixation but is sensitive to organic solvent-containing phospho-specific fix and perm buffers (e.g. True-Phos™). The long-term stability of antibody conjugates can be predicted by its brightness, and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel. Learn more about our flow cytometry flow cytometry buffers.
Heat Stability
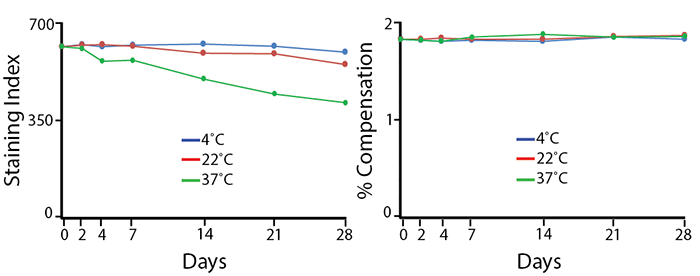
Antibodies were aliquoted into a sealed vial to avoid evaporation and stored at 4°C, 22°C, or 37°C for up to 28 days. The long-term stability of antibody conjugates can be predicted by its brightness (staining index, left) and in the case of PE, PerCP and APC tandems, the percent compensation into the donor channel (right). PE/Fire™ 700 exhibits a reduction of 12% in staining index over 28 days at room temperature (22°C) and 36% reduction in staining index at 37°C. There is no change in compensation and the loss of staining index is due to an increase in non-specific binding of the negative population, not a loss of fluorescence intensity.
Note: Percent compensation for each experiment was obtained on a 5-laser Cytek® Aurora by measuring percent spillover from the tandem dye into the donor fluor (PerCP, PE, or APC) peak emission channel. Percent spillover and compensation values on a conventional cytometer may differ significantly.
 Login/Register
Login/Register 
















Follow Us