Flex-T™ Tetramer Preparation and Flow Cytometry Staining Protocol
Background
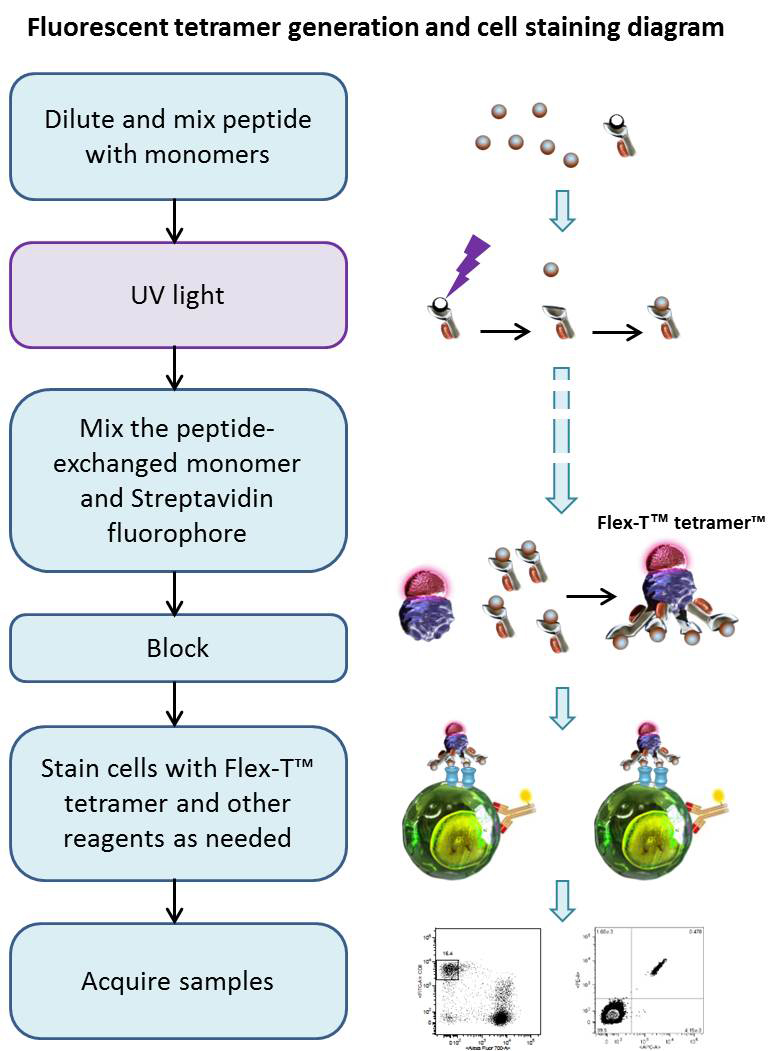
Using UV-induced peptide exchange, MHC/peptide monomers can be generated with conditional Flex-T™ monomers that harbor peptides of interest in their binding grooves. These new MHC monomers are subsequently multimerized using streptavidin-fluorophore conjugates. The resulting Flex-T™ reagents can be used for staining antigen-specific T cells and flow cytometric analysis. In humans, the MHC molecules are called HLA (Human Leukocyte Antigen).
Reagents
- Phosphate buffered saline pH 7.4, 10X concentrate (PBS, BioLegend Cat. 926201)
- Peptide Flex-T™ monomer UVX
- DMSO (e.g. Sigma-Aldrich Cat#D5879)
- 50mM D-Biotin (e.g. Thermo Fisher, Cat#B20656)
- 10% (w/v) NaN3 (e.g Sigma, Cat#S2002)
- Fluorophore-conjugated Streptavidin (BioLegend Cat. 405203, Cat. 405207, Cat. 405225 or equivalent)
- Cell Staining Buffer (BioLegend Cat. 420201 or equivalent)
- 96-well Polypropylene Microplate, V-shape (e.g. Greiner bio-one cat. # 651201)
- 96-well Polystyrene Microplate, U-shape (e.g. Falcon Cat#353077) or 5mL, 12 x 75mm tubes (e.g. Falcon Cat# 352008)
- Plate sealers (BioLegend Cat. 423601)
- 1.5ml tubes (e.g. Eppendorf Cat# 022364111)
- For proteogenomic applications, compatible with our TotalSeq™ product line, use one of our oligo barcoded fluorophore-conjugated Streptavidin reagents
Equipment
- UV Lamp, long-wave UV source (365nm, 8 Watts, or similar). The light source should allow sample to be placed 2 – 5 cm away from the lamp.
- Incubator (37°C)
- Centrifuge capable of accommodating microtiter plates and tubes
- Single and multichannel pipettes capable of accurate delivery of variable volumes, and pipette tips
Tips
- DMSO can be used to dissolve the peptides. However, do not exceed an end concentration of 10% (v/v) in the exchange reaction.
- Avoid repeated freeze-thawing.
- The Flex-T™/peptide solution needs to be kept on ice in the dark as much as possible. Do not work in front of a window.
- The use of short-wavelength (254 nm) or broad-band UV lamps is detrimental to MHC complexes.
- Centrifuge all vials before use (1 minute 2500 x g at 4°C).
Procedure
Peptide Exchange:
- Bring all reagents to 0°C by putting them on ice.
- Dilute 10mM stock solutions of peptides of choice to 400µM by mixing 5µl of peptide stock solution with 120µl PBS, and keep on ice.
- Add 20µl diluted peptide (400µM) and 20µl peptide Flex-T™ monomer UVX (200µg/ml) into 96-well V bottom plate. Mix by pipetting up and down.
- Seal the plate; centrifuge at 2500xg for 2 minutes at 4°C to collect the liquid down
- Remove the seal; put the plate on ice and illuminate with UV light for 30 minutes (the distance of the UV lamp to the samples should be 2-5cm).
- Seal the plate; incubate for 30 minutes at 37°C in the dark.
- To evaluate the efficiency of the peptide exchange follow the Protocol for HLA class I ELISA to evaluate peptide exchange.
Generation of Tetramers:
- Transfer 30µl of peptide-exchanged monomer into a 1.5ml Eppendorf tube, or a new plate, then add 3.3µl of conjugated streptavidin, mix by pipetting up-and-down. Incubate on ice in the dark for 30 minutes. This is enough for about 15 tests.
Note: BioLegend fluorophore-conjugated streptavidin products are recommended. For 30µl of exchanged Flex-T™ monomer we suggest using 3.3µl of BioLegend PE-streptavidin (Cat#405203) or APC- streptavidin (Cat#405207). For BV421-streptavidin conjugate (Cat#405225) use 1.3µl. For oligo barcoded Streptavidin reagents please use 1.3µl. For optimal reaction with other fluorophore-conjugated streptavidin products ensure that the monomer:streptavidin conjugate has a 5:1 ~ 6:1 molar ratio.
(Note that purified, biotinylated, HRP, MojoSort™, and Ultra Streptavidin (USA) kits are not recommended for this procedure.) - During the incubation, prepare blocking solution by adding 1.6µl 50mM D-Biotin and 6µl 10% (w/v) NaN3 to 192.4µl PBS, mix by vortexing. After the incubation, add 2.4µl of blocking solution and pipette up-and-down to stop the reaction.
- Incubate the tubes or sealed plates at 2-8°C overnight (or on ice for 30 minutes in the dark, if staining needs to be performed immediately).
Tip: We recommend Flex-T™ to be assembled with two different streptavidin conjugates in separate reactions. This allows for two-color staining with the same tetramer allele, ensuring the highest specificity.
Cell Staining and Flow Cytometric Analysis:
- Prepare cells of interest
- Prior to performing staining, centrifuge the assembled tetramers in tube or plate at 2500xg for 5 minutes at 4°C. Then keep on ice in the dark.
- Add 2 x 106 cells to 12 x 75mm tubes or a 96-well U-bottom plate. Adjust volume to 200µl with Cell Staining Buffer. Add 2 µl of the assembled tetramers (prepared in Steps 7-9) to each sample, drawing from the top of the solution to avoid any pellet or precipitated aggregates. Mix and incubate on ice in the dark for 30 minutes.
- If co-staining with surface antibodies, prepare an antibody cocktail based on the optimal staining concentration of each reagent. Without washing the cells, add the antibody cocktail directly to the cells containing tetramers. Mix and incubate on ice in the dark for an additional 30 minutes.
- Wash the cells with Staining Buffer two times. Resuspend cells with Staining Buffer.
- Acquire the samples with a flow cytometer and appropriate settings within 2 hours.
Tip: A titration of the Flex-T™ is recommended for optimal performance.
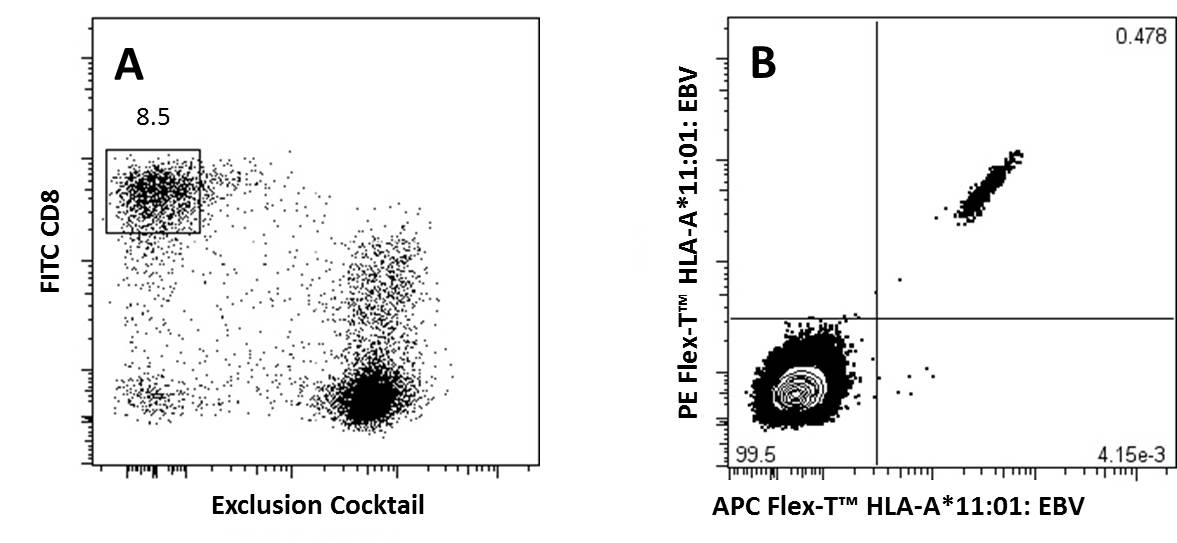
Representative Data:
A) CD8+ T Cells previously gated on lymphocytes (FSC vs SSC) and 7-AAD negative events, were stained with FITC anti-CD8a and an exclusion cocktail containing Alexa Fluor® 700 anti-CD4, CD19, CD14, and CD16.
B) Antigen specific CD8+ T Cells, gated as described, were detected with Flex-T™ tagged with PE and APC. HLA-A*11:01 Flex-T™ was loaded with an EBV peptide (IVTDFSVIK).
Chart Protocol

 Login/Register
Login/Register 







Follow Us