This protocol is intended for use with the following kits:
- MojoSort™ Human CD81 Microbead Exosome Kit (Cat. No. 480174)
- MojoSort™ Human CD63 Microbead Exosome Kit (Cat. No. 480199)
- MojoSort™ Human CD9 Microbead Exosome Kit (Cat. No. 480198)
Kit Components
- 900 μL MojoSort™ Human Exosome Microbeads
- 25 mL MojoSort™ Exosome Buffer (10X)
Note: Store all undiluted components in 2 - 8°C.
Materials Required But Not Included
- MojoSort™ Magnet for 5 or 14 mL tubes (Cat. No. 480019/480020) or other compatible magnetic separator
- 5 mL (12 x 75 mm) or 14 mL (17 x 100 mm) tubes
- 0.2 µm syringe filter
- Starting sample (human cell culture supernatant/heparin-treated plasma/serum/urine)
- Reagents and instruments for sample preparation and analysis
- Optional for releasing exosomes from beads:
0.1 M glycine-HCl (pH 2.2) and 1 M Tris-HCl (pH 9.1)
Important Note
MojoSort™ Human Microbead Exosome Kits can be used with other commercially available free-standing magnets. MojoSort™ protocols are optimized for the MojoSort™ separator and may need to be adjusted for other systems. Please contact BioLegend Technical Service for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Product description and procedure summary:
This product is intended for the separation of human exosomes from cell culture medium, urine, serum, or heparin-treated plasma. Following sample preparation to remove debris, exosome separation is achieved by incubating the sample with MojoSort Human CD81/CD63/CD9 Exosome Microbeads (depending on the kit). The magnetically labeled exosome fraction is retained by the use of a magnetic separator. This magnetically labeled fraction contains the human exosomes of interest; do not discard. Meanwhile, the unmagnetized supernatant contains the unlabeled fraction and may be discarded or collected (if desired for controls). The positively selected bead-bound exosomes may be characterized by downstream applications such as western blot, flow cytometry and qRT-PCR. The bead-bound exosomes may also be released from the beads under acidic conditions (see “Elution of Exosomes” section in the protocol).
Sample Preparation Protocol
Please follow the appropriate sample preparation steps below based on your starting material (cell culture medium, heparin-treated plasma, serum or urine). Sample preparation steps are intended to remove unwanted debris and larger extracellular vesicles.
Cell Culture Medium
- When cells reach ~70-80% confluency, gently wash cells twice with serum-free culture media. If cells of interest grow in suspension, the cells may need to be spun down for wash steps.
- Add serum-free media to the cells and allow them to condition the media for 48 hours.
- Collect the conditioned media after 48 hours and transfer to a fresh tube.
- Centrifuge the conditioned medium at 1200 x g for 10 minutes at 4°C to remove cells and cell debris.
- Transfer the supernatant (avoid any visible pellet) to a new tube.
- Centrifuge the supernatant at 10,000 x g for 30 minutes at 4°C to remove larger undesired extracellular vesicles. Do not discard the supernatant.
- Gently collect the supernatant and filter into a new conical tube using a 0.2 μm pore size filter to remove any debris.
- Aliquot and store the supernatant at -80°C (up to 3 months) until you are ready to proceed with the exosome kit. We recommend a minimum of 0.5 mL cell culture supernatant per test when proceeding with exosome separation using this kit.
- Thaw the cell culture supernatant completely on ice before continuing with the exosome kit.
Optional: Conditioned medium can be concentrated using a 30 kD or 100 kD centrifugal filter tube and the retentate can be used for exosome separation.
Heparin-treated Plasma
- Collect whole blood sample for plasma separation in a heparin-coated anticoagulant tube.
- Centrifuge the blood at 2500 × g for 15 minutes at 4°C and collect the plasma (top layer) in a fresh tube without disturbing the buffy coat.
- Repeat step 2 on the collected plasma fraction to fully eliminate platelets and transfer the platelet-free plasma (PFP) layer into a new tube.
- Centrifuge PFP at 10,000 x g for 30 minutes at 4°C to remove cellular debris and large vesicles. Gently collect the desired supernatant and apply onto 0.2 µm filter into a new tube.
- Aliquot and store at -80°C until ready to proceed with the exosome kit. We recommend a minimum of 0.5 mL of PFP per test when proceeding with exosome separation using this kit.
- Thaw completely on ice before continuing with the exosome kit.
Serum
- Collect whole blood sample for serum separation into a tube without any anticoagulant.
- Allow the blood to clot for at least 30 minutes at room temperature.
- Centrifuge the blood at 2000 × g for 15 minutes at 4°C and collect the upper pale-yellow layer of serum into a new tube.
- Repeat step 3 and transfer the supernatant into a new tube.
- Centrifuge the serum sample at 10,000 x g for 30 minutes at 4°C to remove cellular debris and large extracellular vesicles. Your desired exosomes are in the supernatant so do not discard it.
- Gently collect the desired supernatant and apply onto a 0.2 µm filter into a new tube.
- Aliquot and store at -80°C until ready to proceed with exosome separation. We recommend a minimum of 0.5 mL of serum sample per test when proceeding with exosome separation using this kit.
- Thaw completely on ice before continuing with the exosome separation procedure.
Urine
Note: If using frozen urine, thaw fully on ice before processing the sample. For fresh urine, add protease inhibitors to prevent protein degradation.
- Gently mix the urine sample to obtain a homogeneous suspension.
- Centrifuge the urine sample at 1000 x g for 10 minutes at room temperature.
- Gently remove the supernatant and transfer to a new tube.
- Centrifuge the urine supernatant at 10,000 x g for 30 minutes at room temperature to remove unwanted debris and larger extracellular vesicles.
- Gently collect the desired supernatant and apply onto a 0.2 μm filter into a new tube.
- Aliquot and store at -80°C until ready to proceed with exosome separation. We recommend a minimum of 0.5 mL of urine sample per test when proceeding with exosome separation using this kit.
Optional: Urine sample can be concentrated using a 30 kD or 100 kD centrifugal filter tube and the retentate can be used for exosome separation.
Exosome Separation Protocol
This protocol is written for a single test using a starting volume of 0.5 mL of human biofluids from the sample preparation steps above. For processing larger sample amounts, scale up the volumes accordingly unless otherwise noted. For processing samples < 0.5 mL of biofluids, please bring up the starting sample volume to 0.5 mL using 1X Exosome Buffer and adhere to indicated volumes for a 0.5 mL test according to the protocol below. Please note that using less than the recommended volume of 0.5 mL of biofluids may result in reduced exosome yield.
- Starting sample type: Human cell culture medium, heparin-treated plasma, serum and urine
- Starting sample minimum volume: 0.5 mL
- Starting sample maximum volume: 8 mL
Buffer Preparation
Prepare 1X Exosome Buffer by adding 4.5 mL of distilled water to 0.5 mL of Exosome Buffer (10X). The resulting 5 mL of 1X Exosome Buffer is sufficient for exosome separation from one 0.5 mL unit of starting material.
Bead Preparation
Required tubes:
- 5 mL round bottom tubes (12 x 75 mm) when the starting sample is 0.5 - 3.5 mL.
- 14 mL round bottom tubes (17 x 100 mm) when the starting sample is 3.5 - 8.0 mL.
- Vortex the Microbeads to resuspend the beads completely.
- Depending on your sample volume, choose the appropriate tube size.
- For 5 mL round bottom tubes, add 1 mL of 1X Exosome Buffer. For 14 mL round bottom tubes, add 2 mL of 1X Exosome Buffer.
Note: Do not scale up 1X Exosome Buffer for this step. The volume used (1 or 2 mL) is determined by the tube size, not sample volume - For every 0.5 mL of sample, you will need 20 µL of the Microbeads. Transfer the 20 µL of microbeads to your round bottom tube containing 1X Exosome Buffer.
- Place the tube in the MojoSort Magnet (Cat. No. 480019) for 3 minutes.
Note: If using the 14 mL MojoSort Magnet (Cat. No. 480020), incubate for 5 minutes - Either gently pour out the buffer or remove using a pipette.
- Take out the tube from the magnet and resuspend the beads again with 1 mL (or 2 mL if using 14 mL tube) of 1X Exosome Buffer.
- Repeat steps 5 and 6 for a total of two microbead washes. Proceed immediately to the next step without letting the beads dry out.
Magnetic Exosome Separation
While working with biological samples, it may be important to keep your samples on ice whenever possible to protect the quality and stability for optimal downstream analysis.
- Add 0.5 mL of your starting sample directly to the pre-washed beads to resuspend them. Mix gently by pipetting up and down a few times.
- Incubate for 30 minutes at room temperature with shaking.
- Place the tube on the magnet for 3 minutes (5 minutes if using 14 mL tube) to separate the labeled exosomes. Gently remove and discard the unlabeled fraction (supernatant) by pouring or using a pipette. Do not discard the magnetized labeled fraction.
- Wash: Remove the tube from magnet and wash the labeled exosomes with 0.5 mL excess of the starting volume using 1X Exosome Buffer (e.g. for 0.5 mL of sample, use 1 mL of 1X MojoSort Exosome Buffer). Scale up accordingly.
- Mix gently by pipetting up and down a few times.
- Place the tube on the magnet for 3 minutes (5 minutes if using 14 mL tube) to separate the labeled exosomes. Gently remove and discard the unlabeled fraction (supernatant) by pouring or using a pipette. Do not discard the magnetized labeled fraction.
- Repeat steps 4-6 for a total of two washes.
- Remove the tube from the magnet.
- Resuspend your labeled exosomes in the appropriate buffer for your downstream analysis. For example, if you plan to perform western blotting on your positively selected exosomes, you may resuspend directly in your desired lysis buffer.
Note: For western blotting, you may resuspend directly in your desired lysis buffer. We recommend lysis using Western-Ready™ Rapid Protein Extraction Buffer (Cat. No. 426305). For immunoassays, we recommend lysis using NP-40 lysis buffer without SDS. - Your bead-bound exosomes are now ready for downstream analysis.
Note: To release the captured exosomes from beads, follow the Elution of Exosomes section below.
Elution of Exosomes
This elution protocol is intended to release captured exosomes from the microbeads with a starting sample of 0.5 mL. Elution steps can be scaled up by adjusting all volumes proportionally according to the starting volume of sample.
- Resuspend your positively selected exosomes (from step 9 of the Magnetic Exosome Separation protocol above) in desired volume of 0.1 M Glycine-HCl (pH 2.2).
- Shake for 5 minutes at room temperature with agitation or periodic tapping.
- Place the tube on the magnet for 3 minutes to separate the magnetic beads from the exosomes. Use a pipette gently touching the bottom of the sample tube to collect the supernatant containing the exosomes.
- Transfer the supernatant containing the exosomes to a new tube. Note the volume of collected supernatant for the next step.
- Neutralize immediately by adding 20% (by volume) of 1 M Tris-HCl (pH 9.1).
Chart Protocol

Outline of MojoSort™ Human Microbead Exosome Kit Protocol.
Sample Data
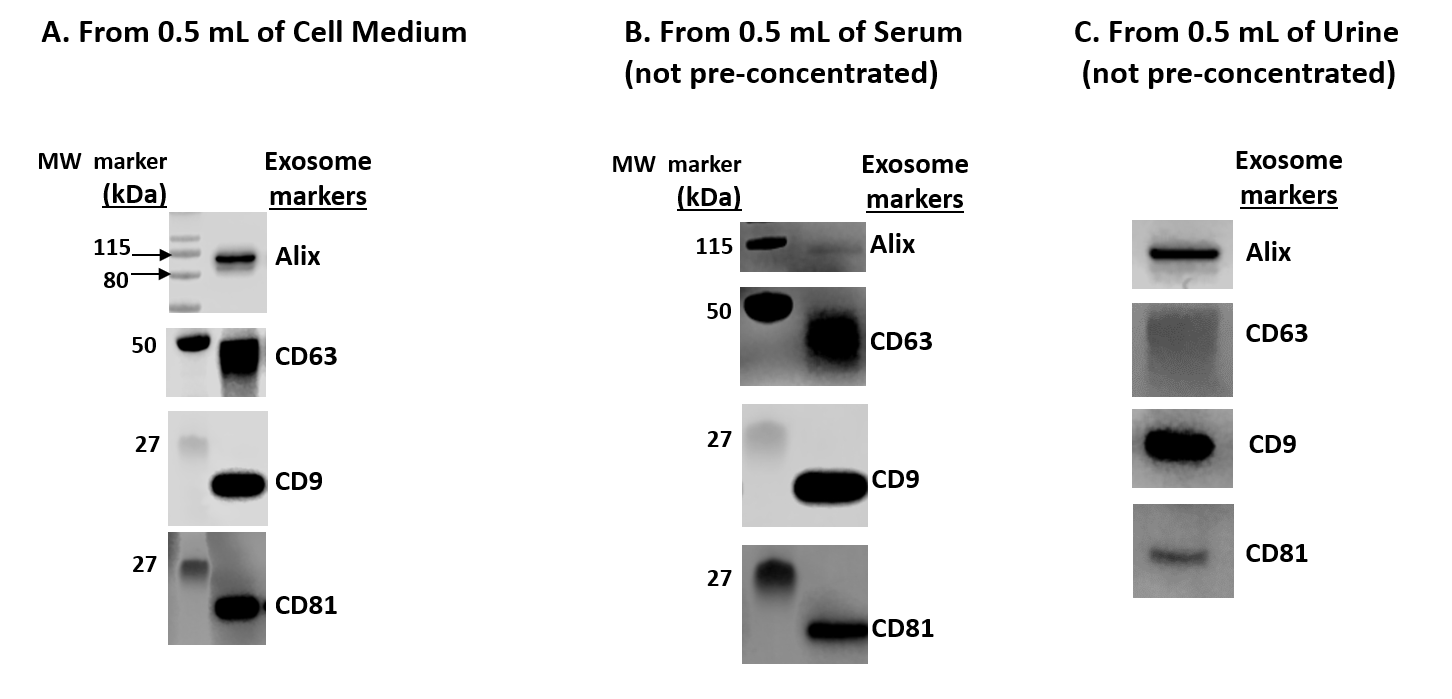
Figure 1. Western Blot Analysis of isolated exosomes using MojoSort™ Human CD81 Microbead Exosome Kit from the indicated human biofluid samples A) 0.5 mL of conditioned HEK293T cell culture medium B) 0.5 mL of human serum C) 0.5 mL of human urine.
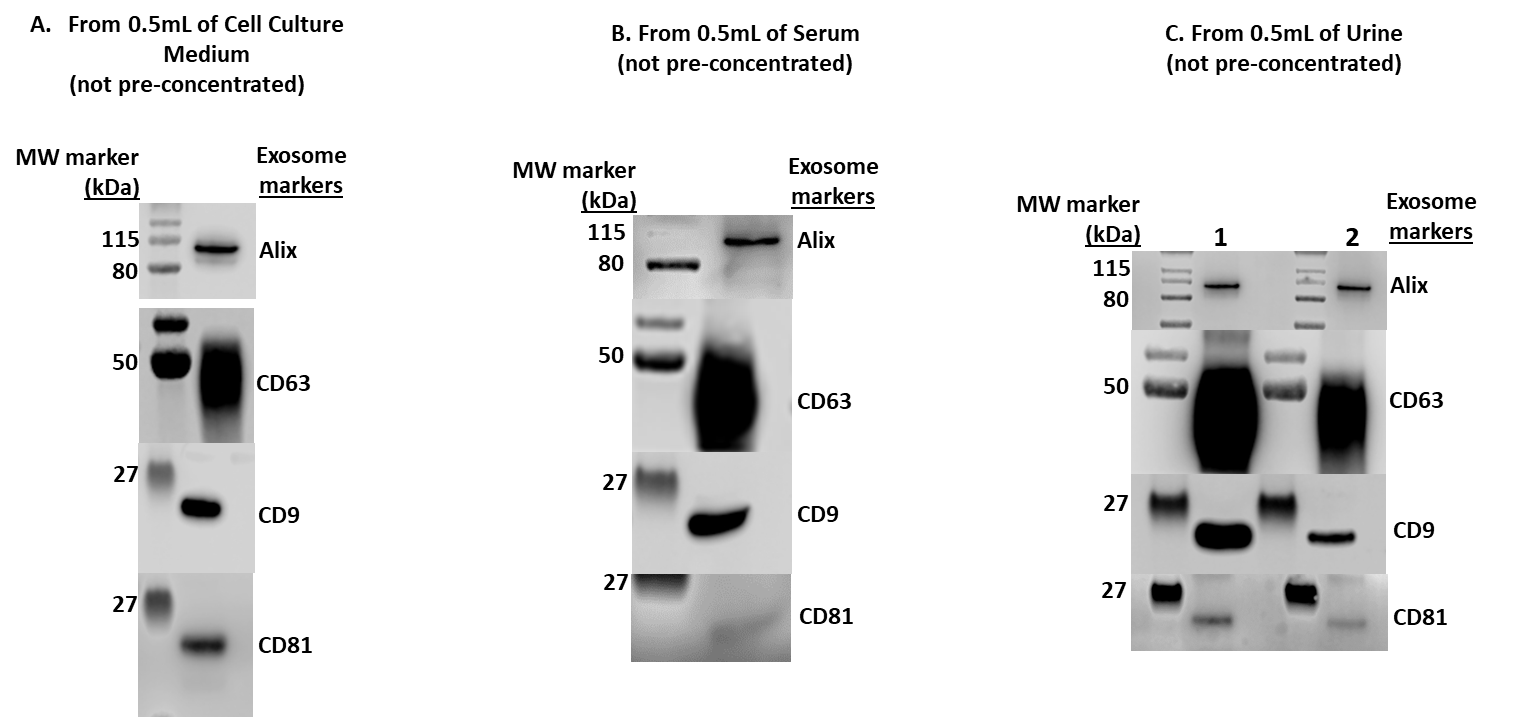
Figure 2. Western Blot Analysis of isolated exosomes using MojoSort™ Human CD63 Microbead Exosome Kit from the indicated human biofluid samples A) 0.5 mL of conditioned HEK293T cell culture medium B) 0.5 mL of human serum C) 0.5 mL of human urine from two different donors (donor 1 and 2).
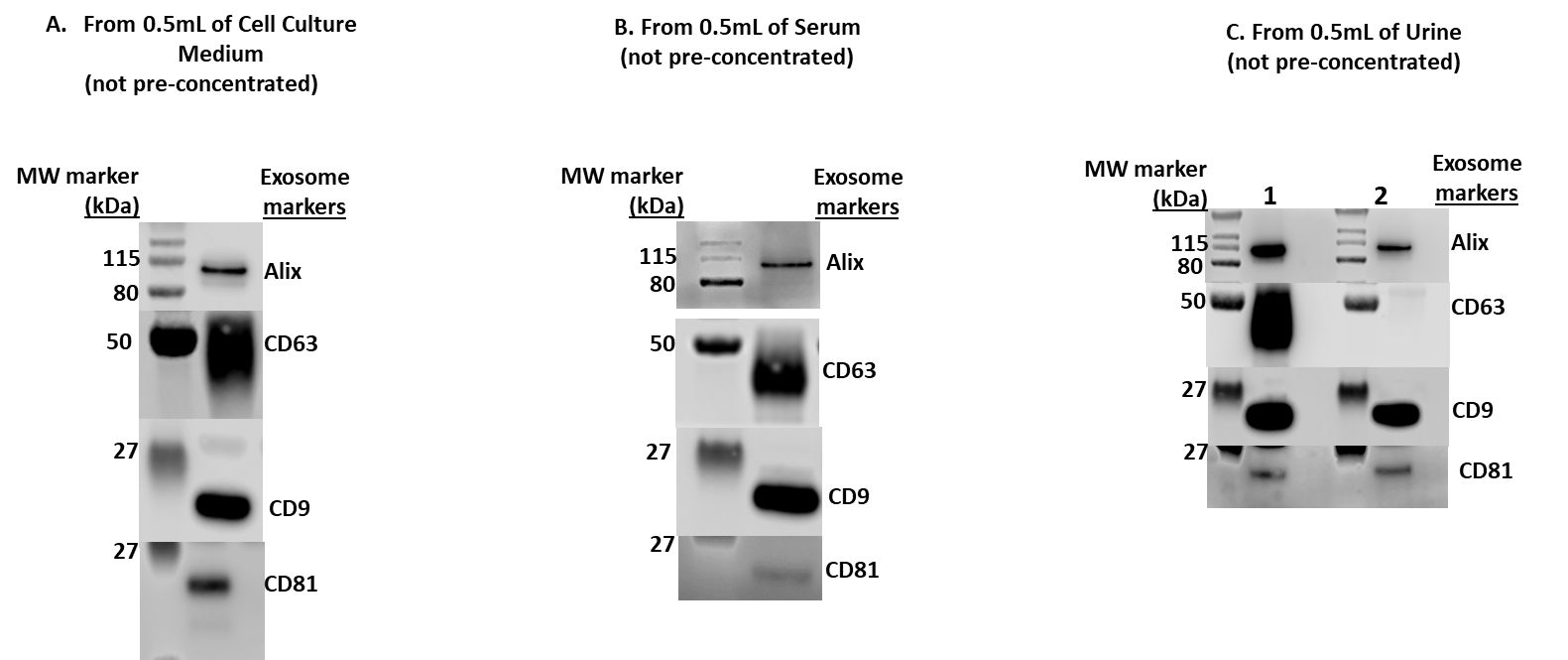
Figure 3. Western Blot Analysis of isolated exosomes using MojoSort™ Microbead Human CD9 Exosome Kit from the indicated human biofluid samples A) 0.5 mL of conditioned HEK293T cell culture medium B) 0.5 mL of human serum C) 0.5 mL of human urine (from two different donors).
Guidance for Western Blot Analysis for Isolated Exosomes
- You may add lysis buffer directly to the labeled exosomes in step 9 of the Magnetic Exosome Separation section. For western blotting, we recommend lysis using Western-Ready™ Rapid Protein Extraction Buffer (Cat. No. 426305).
- You may want to use antibodies against markers of exosomes such as CD9, CD63, CD81, and Alix.
- We highly recommend rabbit monoclonal antibodies for detecting exosome markers due to the capture antibodies being mouse IgG.

 Login/Register
Login/Register 






Follow Us