- Clone
- O95A2 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Sequestosome 1, p62, Phosphotyrosine-Independent Ligand For The Lck SH2 Domain Of 62 KDa, EBI3-Associated Protein Of 60 KDa, Ubiquitin-Binding Protein p62, Oxidative Stress Induced Like, Autophagy Receptor P62
- Isotype
- Mouse IgG1, κ
-
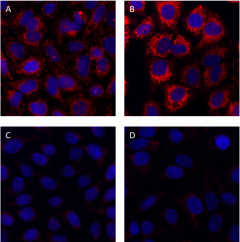
Non-targeting siRNA-treated HeLa cells were grown in normal media (panel A) or media treated with 50 µM chloroquine for 18 hours (panel B). As a negative control, HeLa cells were depleted of p62 (SQSTM1) protein using siRNA directed against SQSTM1 and grown in normal media (panel C) or media treated with 50 µM chloroquine for 18 hours (panel D). Cells were then fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes and blocked with 5% FBS for 60 minutes. Cells were then intracellularly stained with 2.0 µg/mL (1:250 dilution) of purified anti-p62 (SQSTM1) antibody (clone O95A2) overnight at 4°C, followed by incubation with Alexa Fluor® 594 goat anti-mouse IgG antibody (Cat. No. 405326) at 1:200 dilution. Nuclei were counterstained with DAPI and the image was captured with a 60X objective.
| Cat # | Size | Price | Quantity Check Availability | ||
|---|---|---|---|---|---|
| 937201 | 25 µg | $101.00 | |||
| 937202 | 100 µg | $253.00 | |||
In eukaryotes, autophagy plays a critical role in the maintenance of cellular proteostasis. Sequestosome-1 (SQSTM1), or p62 functions as a receptor during selective macroautophagy, where it is responsible for recognition and loading of misfolded proteins and dysfunctional organelles into autophagosomes for cytosolic clearance and recycling. p62 contains a ubiquitin-association (UBA) domain which directly binds ubiquitinated protein aggregate substrates. Under normal conditions, p62 exists as a dimer with the UBA domain contributing to the dimer interface. Upon phosphorylation and acetylation at residues embedded within the UBA, dimerization is disrupted, allowing the UBA to bind ubiquitinated substrates for clearance. In addition to the UBA domain, p62 contains an LC3-interacting domain that facilitates interaction with the autophagosome membrane adaptor proteins LC3 and ATG8. In addition to its role in autophagy, p62 can also function as a scaffold signaling hub and positively regulate the mTORC1, NF-κB, and Keap1-Nrf2 signaling pathways. Dysregulation of p62 and autophagy is associated with a broad spectrum of diseases, including cancer, neurodegenerative, and metabolic diseases.
Product Details
- Verified Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Immunogen
- Full-length recombinant human SQSTM1 protein
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide
- Preparation
- The antibody was purified by affinity chromatography.
- Concentration
- 0.5 mg/mL
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C.
- Application
-
ICC - Quality tested
- Recommended Usage
-
Each lot of this antibody is quality control tested by immunocytochemistry. For immunocytochemistry, a concentration range of 2 - 5 μg/mL is recommended. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
When this clone was tested for western blot, a non-specific band close to the predicted molecular weight of p62 was observed. This protein was also detected in O95A2 immunoprecipitates. We therefore do not recommend this clone for western blot or immunoprecipitation applications.
This clone was tested for ICC using 4% PFA-fixed HeLa cells permeabilized with Triton X-100 or methanol. Both methods were compatible with p62 staining. - RRID
-
AB_2861124 (BioLegend Cat. No. 937201)
AB_2861124 (BioLegend Cat. No. 937202)
Antigen Details
- Structure
- p62 is a 440 amino acid protein with a predicted molecular weight of 47 kD.
- Distribution
-
Vesicles and Cytosol/Ubiquitously expressed
- Function
- Autophagy
- Biology Area
- Cell Biology, Neuroscience, Protein Misfolding and Aggregation
- Molecular Family
- Adaptor Proteins
- Antigen References
-
- Komatsu M, et al. 2007. Cell. 6:1149.
- Bjørkøy G, et al. 2006. Autophagy. 2:138.
- Bjørkøy G, et al. 2005. J Cell Biol. 171:603.
- Gene ID
- 8878 View all products for this Gene ID
- UniProt
- View information about p62 on UniProt.org
Other Formats
View All p62 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Purified anti-p62 (SQSTM1) | O95A2 | ICC |
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Purified anti-p62 (SQSTM1)
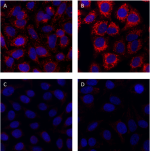
Non-targeting siRNA-treated HeLa cells were grown in normal ...
