- Regulatory Status
- RUO
- Other Names
- Urogastrone (URG), HOMG4
-

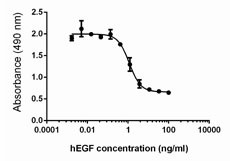
Inhibition of A431 cell proliferation by human EGF.
| Cat # | Size | Price | Quantity Check Availability | ||
|---|---|---|---|---|---|
| 585506 | 100 µg | $89.00 | |||
| 585508 | 500 µg | $218.00 | |||
Epidermal growth factor (EGF) is a small 6 kD polypeptide and has six conserved cysteine residues that form three intramolecular disulfide bonds. Human and mouse EGF share 70% homology in amino acid structure. Human EGF is synthesized as a transmembrane precursor protein (1207 amino acids) which is proteolytically cleaved to generate the 54 amino acid mature EGF. Many different cells including mammary gland cells, macrophages, gut epithelial cells, and cells in the nervous system and the kidney can produce EGF. EGF plays important roles in the regulation of cell survival, proliferation, and differentiation by binding to its receptor EGFR. For example, EGF can stimulate the proliferation of mouse embryonic stem cells or induce the terminal differentiation/growth inhibition of A431 cells. The binding of EGF to EGFR will induce receptor dimerization, which is required for activating the tyrosine kinase in the receptor cytoplasmic domain. In addition, the binding of EGF to its receptor triggers several signal transduction pathways including JAK/STAT, Ras/ERK, and PI3K/AKT pathways. Blocking of the EGF/EGFR pathway can suppress some tumor cell's proliferation. Other members of the EGF family (including transforming growth factor-α (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin (AR), betacellulin (BTC), epiregulin (EPR), and epigen also bind to EGFR.
Product Details
- Source
- Human EGF, 54 amino acids Asn971-Arg1023 with an N-terminal Met (Accession# P01133) was expressed in E. coli.
- Molecular Mass
- The 54 amino acid recombinant protein has a predicted molecular mass of approximately 6 kD. The DTT-reduced protein migrates at approximately 6 kD and non-reduced protein migrates at approximately 13 kD by SDS-PAGE. The N-terminal amino acid is Methionine.
- Purity
- >98%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in PBS.
- Endotoxin Level
- Less than 0.01 ng per µg cytokine as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- Human EGF inhibits the proliferation of human epithelial A431 cells in a dose-dependent manner. The ED50 for this effect is 0.3 - 2.0 ng/mL.
- Application
-
Bioassay
- Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
-
Application References
(PubMed link indicates BioLegend citation) -
- Henson ES and Gibson SB. 2006. Cell Signal. 18:2089.
- Burgess AW, et al. 2003. Mol. Cell. 12:541.
- Imai Y, et al. 1982. Cancer Res. 42:4394.
- Barnes DW. 1982. J. Cell. Biol. 93:1.
- Heo JS, et al. 2006. Am. J. Physiol. Cell. Physiol. 290:C123.
- Product Citations
-
Antigen Details
- Distribution
-
Mammary gland cell, macrophage, gut epithelial cells, cells in the nervous system, kidney
- Function
- EGF is a potent mitogen for many cells in culture, and in vivo, it induces the proliferation and differentiation of skin, cornea, lung, and trachea, among other tissues. Processing of pro EGF to mature EGF in different tissues is not equally efficient. The precursor is processed to mature EGF in the submaxillary gland, pancreas, small intestine, and mammary gland. In the submaxillary gland, EGF is fully processed, stored at secretory granules, and secreted in saliva. In kidney, EGF is present in unprocessed or intermediated forms on the cell surface.
- Ligand/Receptor
- EGFR
- Cell Type
- Embryonic Stem Cells, Hematopoietic stem and progenitors, Mesenchymal Stem Cells, Neural Stem Cells
- Biology Area
- Cell Biology, Immunology, Innate Immunity, Neuroscience, Stem Cells, Synaptic Biology
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Gene ID
- 1950 View all products for this Gene ID
- UniProt
- View information about EGF on UniProt.org
