- Regulatory Status
- RUO
- Other Names
- Platelet-derived growth factor CC, PDGFCC, PDGF-CC
-

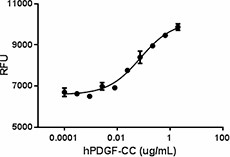
Human PDGF-CC induces proliferation of NIH3T3 mouse embryonic fibroblast cells in a dose dependent manner with ED50 of 0.05 - 0.2 µg/mL.
| Cat # | Size | Price | Quantity Check Availability | ||
|---|---|---|---|---|---|
| 766702 | 5 µg | $89.00 | |||
| 766704 | 20 µg | $218.00 | |||
This product is not available for shipping outside of the United States.
Select size of product is eligible for a 40% discount! Promotion valid until December 31, 2024. Exclusions apply. To view full promotion terms and conditions or to contact your local BioLegend representative to receive a quote, visit our webpage.
Platelet-derived growth factors (PDGFs) are homo- or heterodimers of four polypeptides, known as A, B, C and D chains. PDGF was initially discovered as a major mitogenic factor present in serum but absent from plasma. Five PDGF isoforms (AA, BB, AB, CC and DD) have been identified. The dimeric isoforms of PDGFs are differentially expressed in various cell types and their effects are mediated through distinct dimeric receptors made up of two structurally similar protein-tyrosine kinase receptor subunits (αα-, αβ-, or ββ-PDGFR). PDGF-AA, AB, and BB dimers are processed intracellularly and secreted as active dimers that readily activate PDGF receptors. PDGF-CC and DD are secreted as full-length, latent dimers, and the proteolytic removal of a CUB domain is required for the growth factor domain of PDGF-CC or DD to activate the PDGF receptors. PDGFs are potent mitogens for connective tissue cells, including dermal fibroblasts, glial cells, arterial smooth muscle cells and some epithelial and endothelial cells. PDGFs usually act primarily in paracrine manner and may be engaged in autocrine loops in tumors. In addition to its activity as a mitogen, PDGF is chemotactic for fibroblasts, smooth muscle cells, neutrophils and mononuclear cells.
Product Details
- Source
- Human PDG-CC, amino acids Val235-Gly345 (Accession # NP_057289) was expressed in E. Coli.
- Molecular Mass
- The 112 amino acid recombinant proteins has predicted molecular mass of approximately 12.6 kD. The predicted N-terminal amino acid is Met.
- Purity
- >98%, as determined by SDS-PAGE gel and HPLC analysis.
- Formulation
- Lyophilized from 0.2 µm filtered protein solution in 5 mM sodium citrate.
- Endotoxin Level
- Less than 1 EU per µg protein as determined by the LAL method.
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C for one year. For maximum results, quick spin vial prior to opening. Reconstitute in water to a concentration of 0.1-0.5 mg/ml. Reconstituted samples can be kept at 4°C for one week and six months at -20°C or -70°C. Do not vortex. It is recommended to further dilute in a buffer containing a carrier protein such as 0.1% BSA and store working aliquots at -20°C or -70°C. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 0.05 – 0.2 µg/mL as measured by the ability of protein to induce proliferation of NIH3T3 mouse embryonic fibroblast cells. Deep Blue Cell Vialibity Kit was used to quantitate cell proliferation.
- Application
-
Bioassay
Antigen Details
- Structure
- Homodimer
- Distribution
-
Platelets, Monocytes, Macrophages, Mast Cells
- Function
- Growth factor that plays an essential role in the regulation of embryonic development, cell proliferation, cell migration, survival and chemotaxis. Potent mitogen for cells of mesenchymal origin.
- Interaction
- Platelets, Fibroblast, Endothelial cells, Epithelial cells, Muscle cells, Neurons, Astrocytes, Oligodendrocytes, Neutrophils
- Ligand/Receptor
- PDGFR-αα, PDGFR-αβ
- Bioactivity
- Measured by its ability to induce proliferation of NIH3T3 cells.
- Biology Area
- Angiogenesis, Cell Biology, Immunology, Neuroscience, Neuroscience Cell Markers, Stem Cells
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
1. Heldin CH and Westermark B. 1999. Physiol. Rev. 79:1283.
2. Fredriksson L, et al. 2004. Cytokine Growth Factor Rev. 15:197.
3. Hu JG, et al. 2012. J. Mol. Neurosci. 46:644.
4. Schneider L, et al. 2010. Cell Physiol. Biochem. 25:279.
5. Karlsson C and Paulsson Y. 1994. J. Cell Physiol. 158:256.
6. Shure D, et al. 1992. Biochem. Biophys. Res. Commun. 186:1510.
7. Carlin SM, et al. 2003. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L1020
8. Ustach CV, et al. 2005 Mol. Cell Biol. 25:6279-6288.
9. Jones AV and Cross NC. 2004. Cell Mol. Life Sci. 61:2912-23.
10. Andrae J, et al. 2008. Genes Dev. 22:1276-312. - Gene ID
- 56034 View all products for this Gene ID
- UniProt
- View information about PDGF-CC on UniProt.org
