- Regulatory Status
- RUO
- Other Names
- IL-12 p80, IL-12 subunit p40, IL-23 subunit p40, Cytotoxic lymphocyte maturation factor, CLMF2, Natural killer cell stimulatory factor 40-KD subunit, NKSF2
-

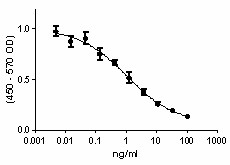
Mouse IL-12 p40 homodimer is able to inhibit IL-12-dependent IFNγ production in splenocytes.
| Cat # | Size | Price | Quantity Check Availability | ||
|---|---|---|---|---|---|
| 573102 | 10 µg | $206.00 | |||
| 573104 | 25 µg | $382.00 | |||
IL-12 and IL-23 share the p40 subunit, which heterodimerizes respectively with IL-12 p35 or IL-23 p19 subunits to form IL-12 or IL-23. IL-12 p40 exists as a monomer and as a homodimer (IL-12 p80). IL-12 induction is relevant in asthmatic airway inflammation. IL-12 expression can be induced by mouse parainfluenza type I (Sendai) virus and its source is airway epithelial cells. In that experimental model, IL-12 induction is followed by excessive expression of IL-12 p40 that could be further enhanced in IL-12 p35-deficient mice. Overexpression of IL-12 p80 causes macrophage accumulation and contributes to airway inflammation and consequent morbidity during viral bronchitis. Amplified epithelial IL-12 p40 expression and augmented concentrations of BAL fluid IL-12 p40 (but not IL-12 p70) has been detected in asthmatic subjects. It has been demonstrated that p80, but not IL-12 or p40, induces macrophage chemotaxis that is independent of IL-12 and mediated through the cytoplasmic tail of IL-12b1. Additional studies with transgenic mice suggest that overexpression of IL-12 p80 prior to a viral infection increases the number of resident airway macrophages, and this primes the host for a protective response against a lethal respiratory viral infection. In addition, it has been suggested that p80 functions as a competitive antagonist of IL-12 p70. Mouse Con A-activated splenocytes display identical binding affinities for p80 and IL-12, and in these cells p80 competitively inhibited IL-12 binding and IL-12-dependent proliferation. Furthermore, p80 is able to inhibit IL-12-dependent IFNγ production in freshly isolated splenocytes.
Product Details
- Source
- Mouse IL-12 p40 homodimer, amino acids Met23-Ser335 (Accession # NM_008352), was expressed in insect cells.
- Molecular Mass
- The 313 amino acid recombinant protein has a predicted molecular mass of 35.8 kD. The DTT-reduced protein migrates at approximately 40 kD and the non-reduced protein migrates at approximately 75 kD by SDS-PAGE. The N-terminal amino acid is Met.
- Purity
- >98%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in 20 mM Tris-HCl, pH 8.0, 0.1 M NaCl
- Endotoxin Level
- Less than 0.01 ng per µg cytokine as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 100 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 1- 4 ng/ml corresponding to a specific activity of 1.0 - 0.25 x 106 units/mg, as determined by the dose dependent inhibition of IL-12-dependent IFNγ production in splenocytes.
- Application
-
Bioassay
- Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
-
Application References
(PubMed link indicates BioLegend citation) -
- Wang X, et al. 1999. Eur. J. Immunol. 29:2007.
- Walter JM, et al. 2001. J. Exp. Med. 193:339.
- Russell TD, et al. 2003. J. Immunol. 171:6866.
- Mikols CL, et al. 2006. Am. J. Respir. Crit. Care 174:461.
- Gunsten S, et al. 2008. Immunology 126:500.
- Jana M, et al. 2009. Glia 57:1553.
- Yabu M, et al. 2010. Int Immunol. 23:29. PubMed
- Product Citations
-
Antigen Details
- Structure
- Homodimer
- Distribution
-
IL-12 p40 homodimer is produced by airway epithelial cells. The expression level of p40 is much higher than that of p35 in IL-12 p70-producing cells; therefore, monocytes, macrophages, neutrophils, dendritic cells, and B cells might express p40 homodimer.
- Function
- p40 homodimer functions as a proinflammatory protein that enhances leukocyte accumulation in the skin, blunt Th1 immunity to Plasmodium berghei, and provides protective immunity toward mycobacterial infection. In addition, p40 homodimer induces macrophage chemotaxis independent of IL-12. Also, IL-12 p40 homodimer (p402) induces the expression of inducible nitric oxide synthase (iNOS) in microglia.
- Interaction
- Airway epithelial cell, macrophages, microglia, and spleenocytes.
- Ligand/Receptor
- IL-12Rβ1
- Biology Area
- Cell Biology, Immunology, Innate Immunity
- Molecular Family
- Cytokines/Chemokines
- Gene ID
- 3593 View all products for this Gene ID
- UniProt
- View information about IL-12 p40 on UniProt.org
