Spark Violet™ 538
Spark Violet™ 538 slots in as an additional violet laser fluor option for multicolor flow cytometry users. This dye is optimally excited by the 405 nm violet laser and emits maximally at 538 nm, placing its peak emission between Brilliant Violet 510™ and Brilliant Violet 570™. When used on a spectral cytometer, Spark Violet™ 538 can be successfully unmixed from neighboring dyes, such as BV510™, BV570™, and BV605™. While it is brighter than Pacific Orange™, Spark Violet™ 538 is relatively dim and should be paired with antigens expressed at high levels.
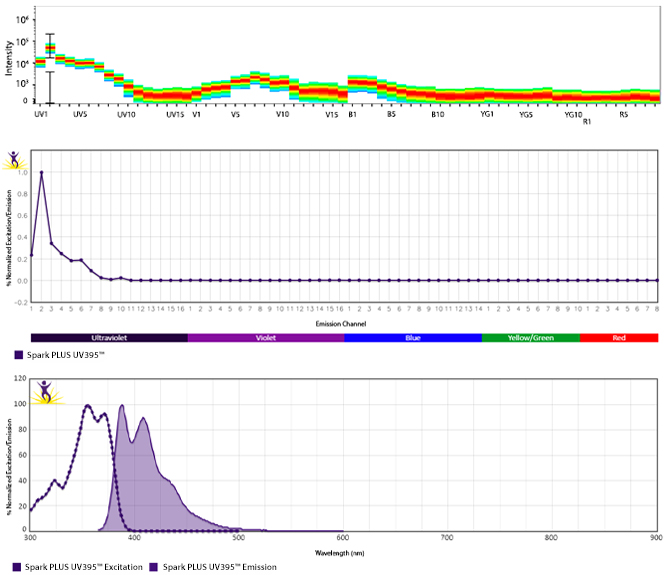
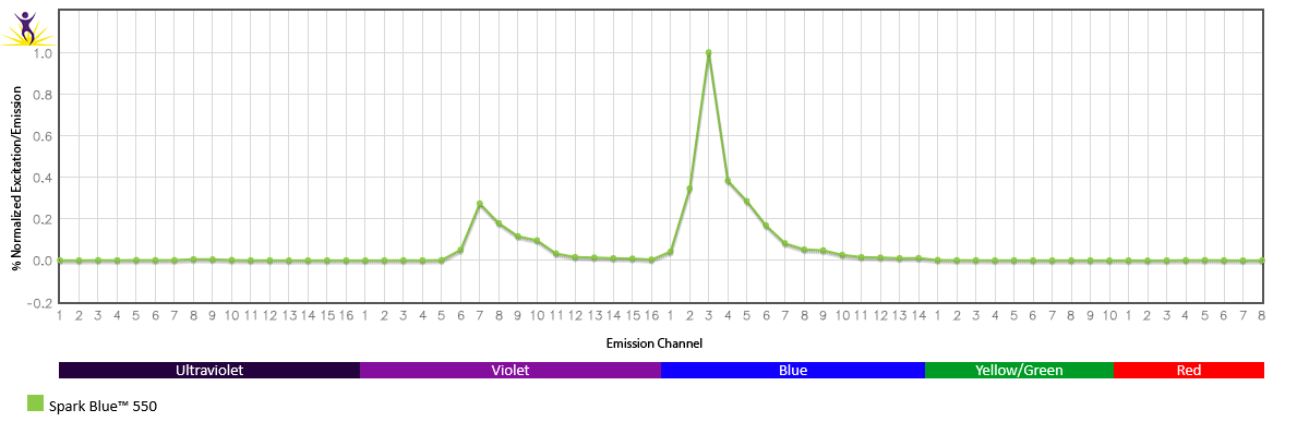
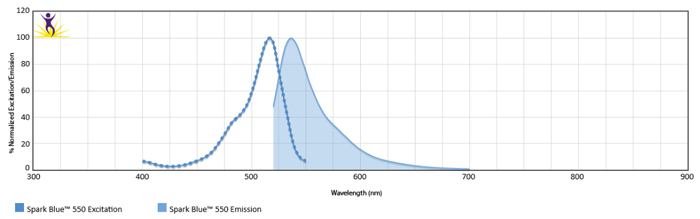
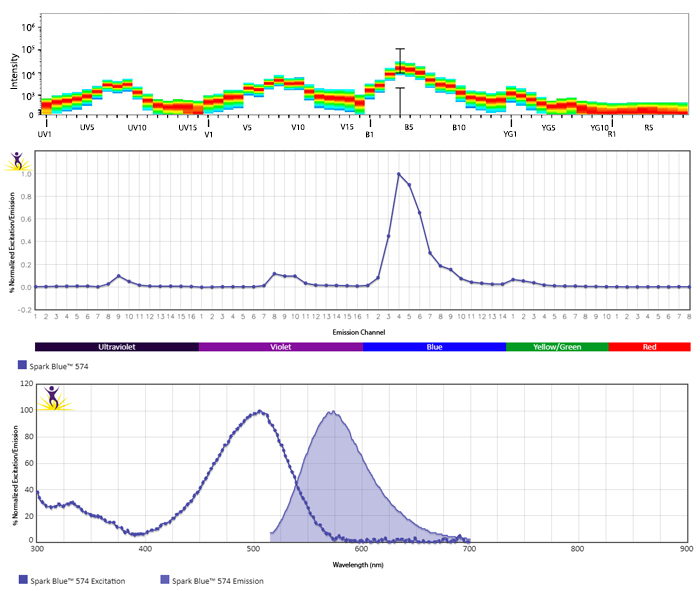
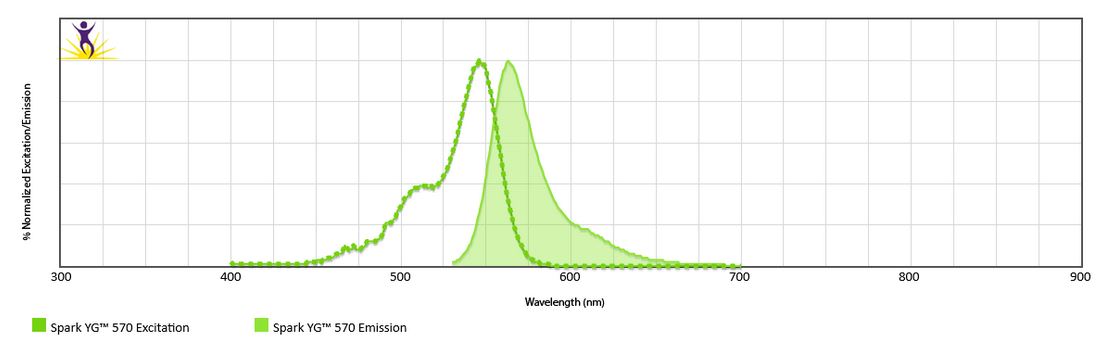
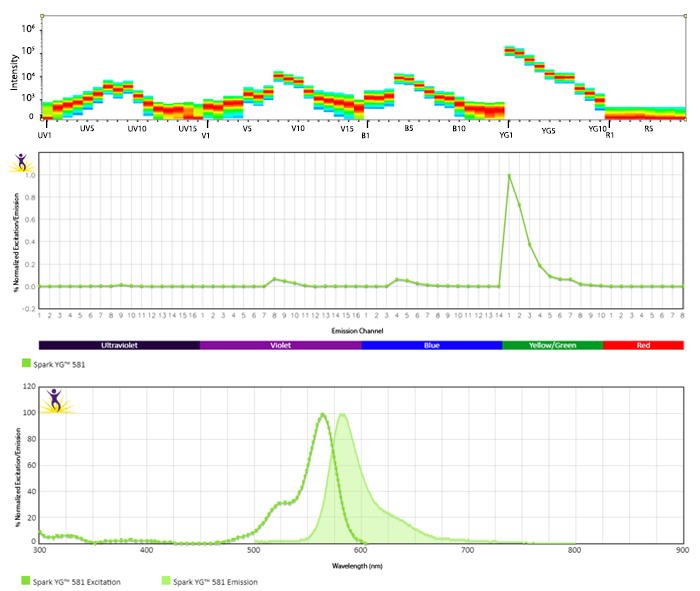
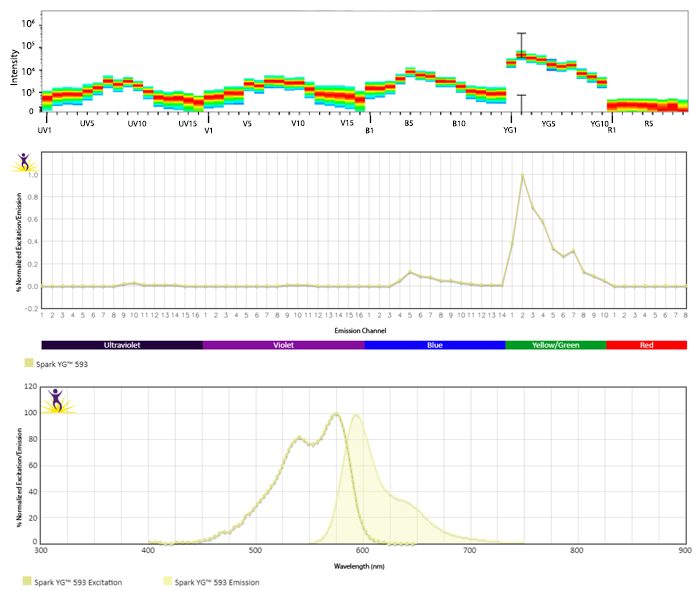
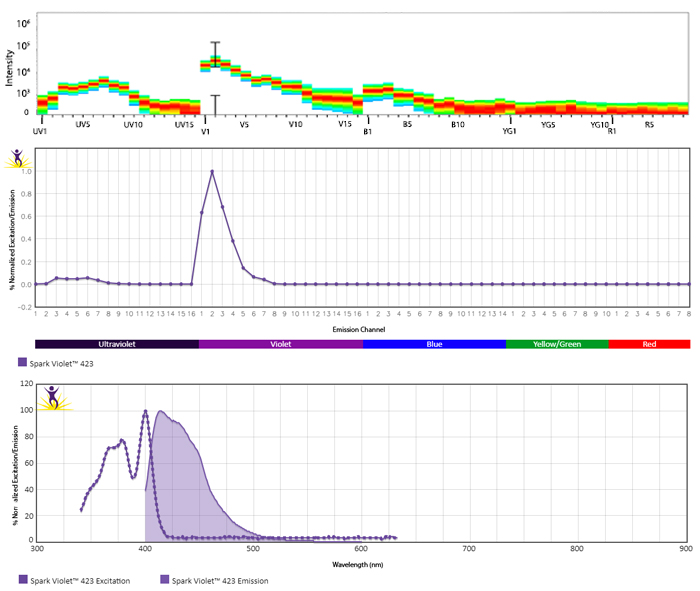
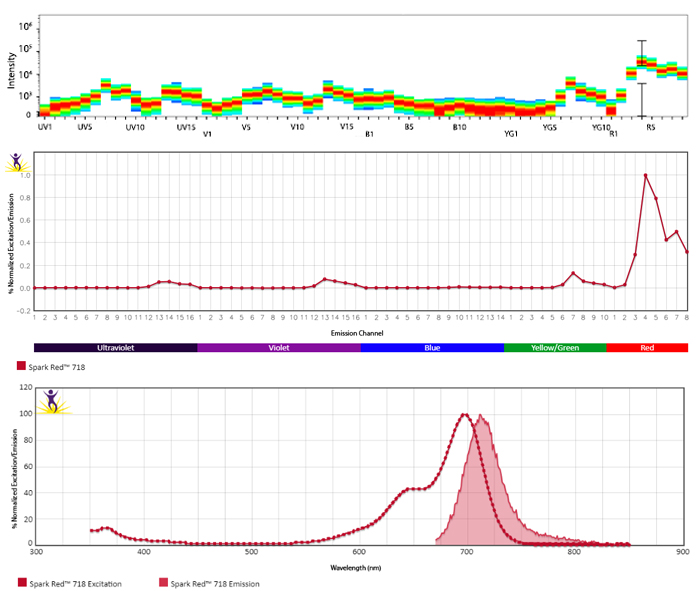
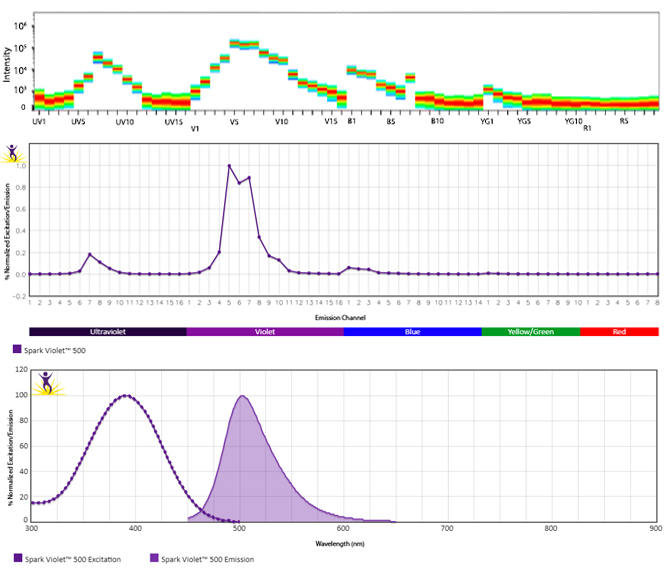
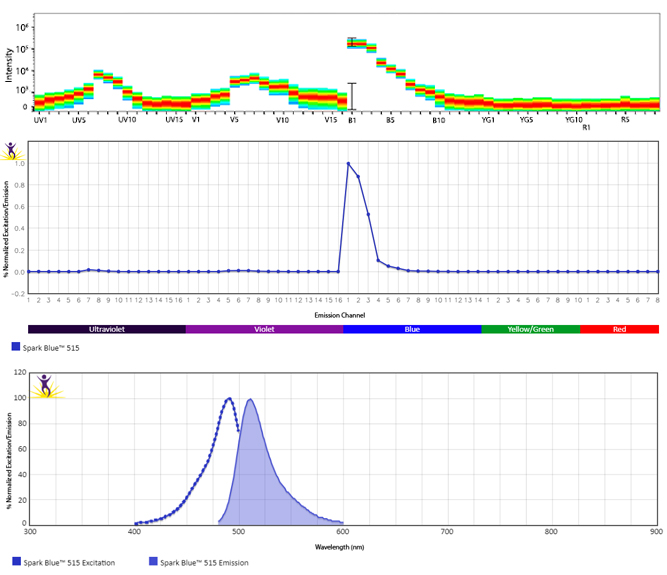
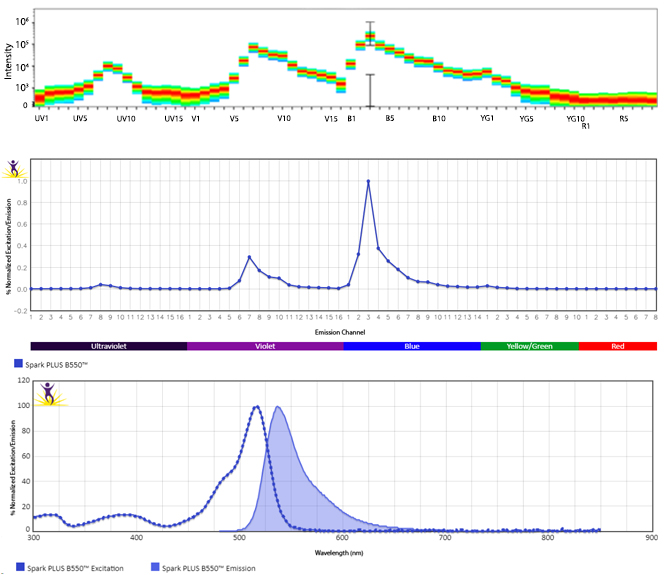
Excitation and Emission Spectra of Spark Violet™ 538
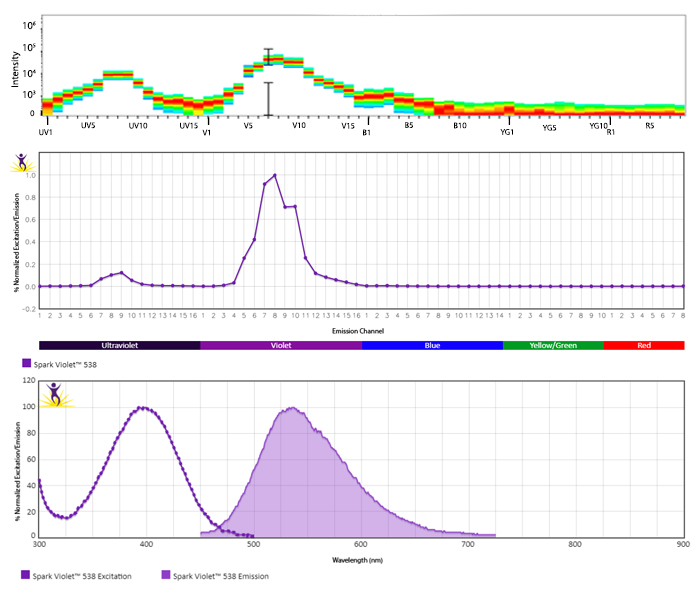
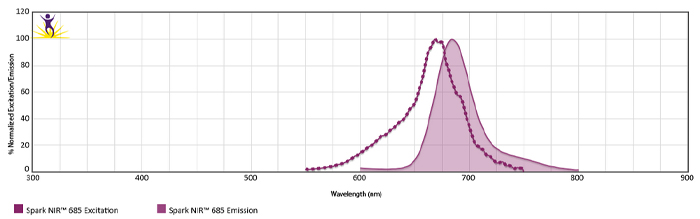
Emission spectra (top) and normalized emission spectra (middle) of Spark Violet™ 538 run on a 5-laser Cytek™ Aurora Spectral Cytometer. To compare Spark Violet™ 538 with other fluorophores on a spectral cytometer, use our Aurora Spectral Analyzer tool.
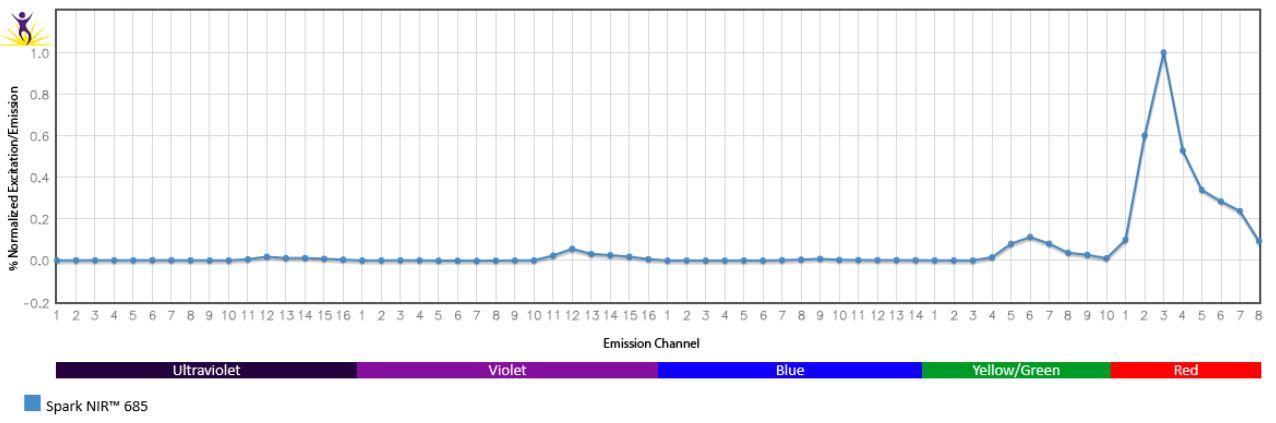
Normalized excitation and emission spectra of Spark NIR™ 685 obtained from a spectrophotometer (bottom). To compare Spark Violet™ 538 with other fluorophores, use our Fluorescence Spectra Analyzer tool.
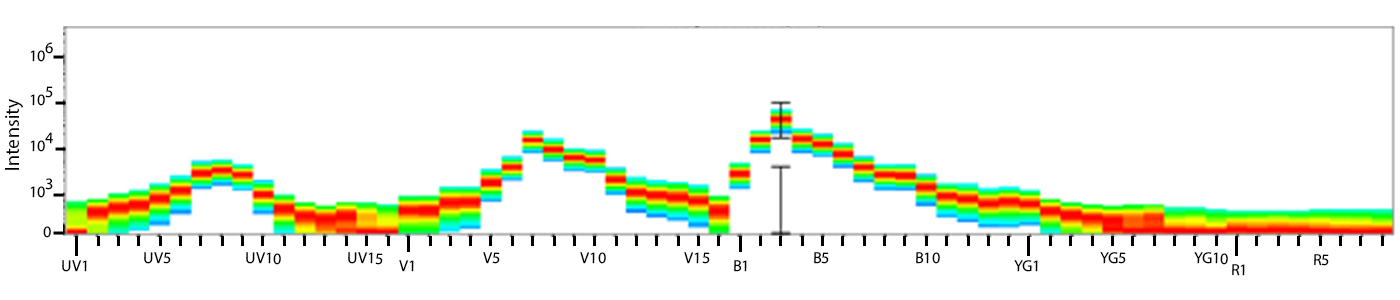
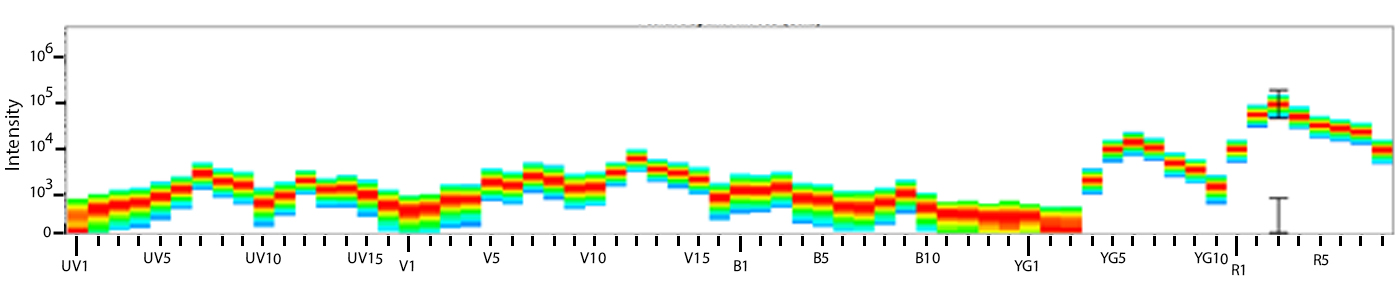
Utilize Spark NIR™ 685 With Other Red Laser Fluorophores
Spark NIR™ 685 can be used in the same panel as APC or Alexa Fluor® 647; however, spreading error with these fluors must be considered during panel design and panels should be built to match proper fluor brightness with antigen expression. For example, in an ideal panel, Spark NIR™ 685 and Alexa Fluor® 647 would not be used to detect two co-expressed markers.
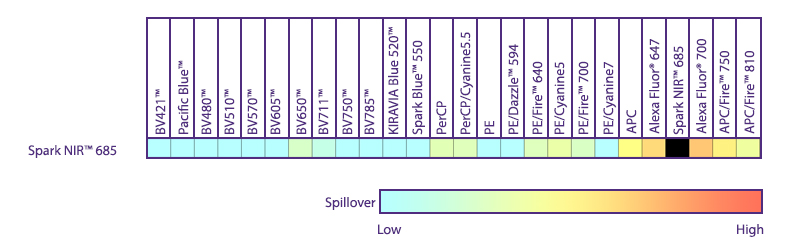
Spreading impact of Spark NIR™ 685 into detection channels of a 5-laser Cytek™ Aurora Spectral Cytometer.
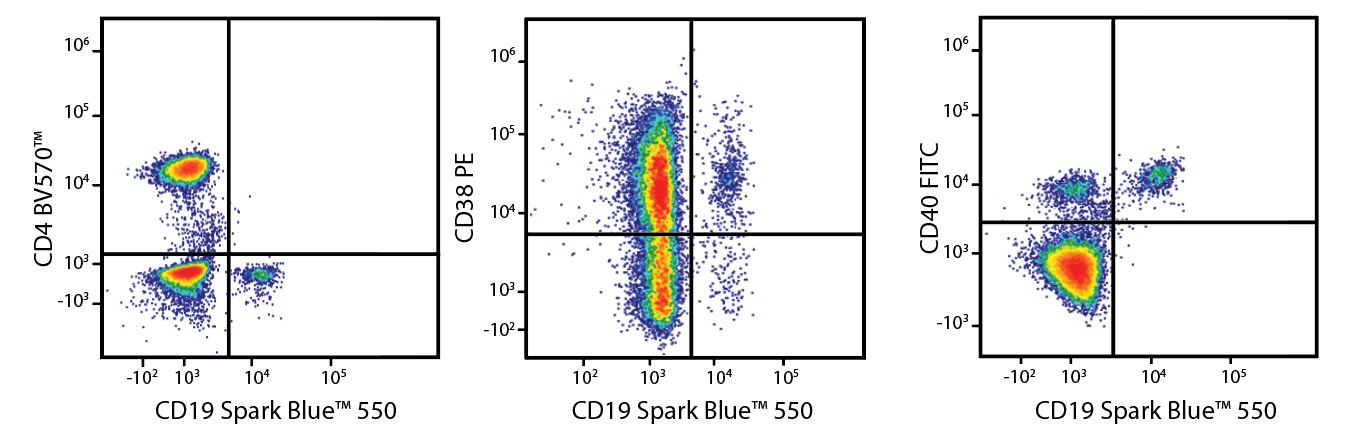
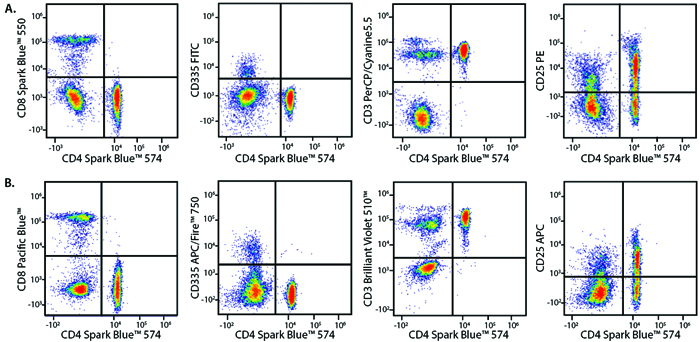
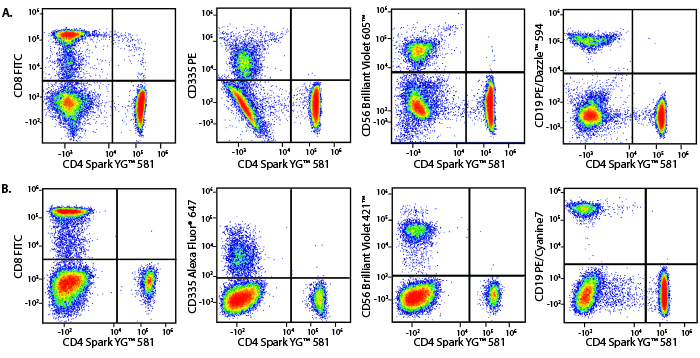
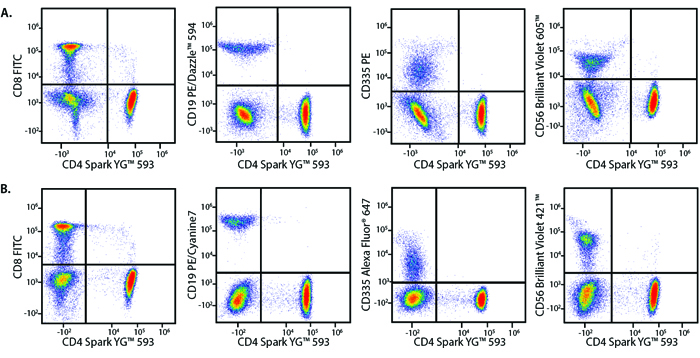
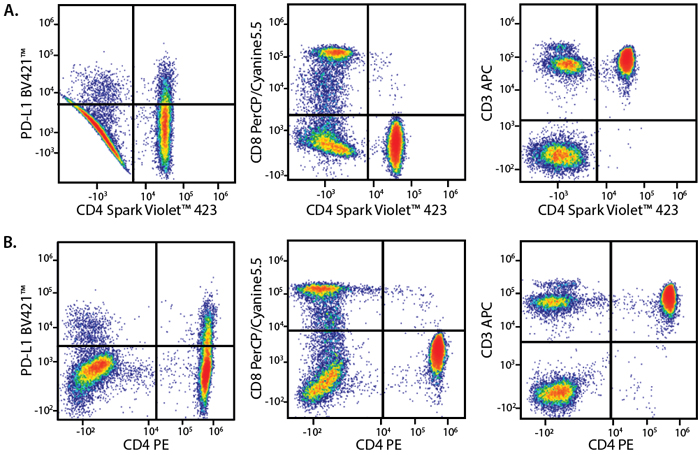
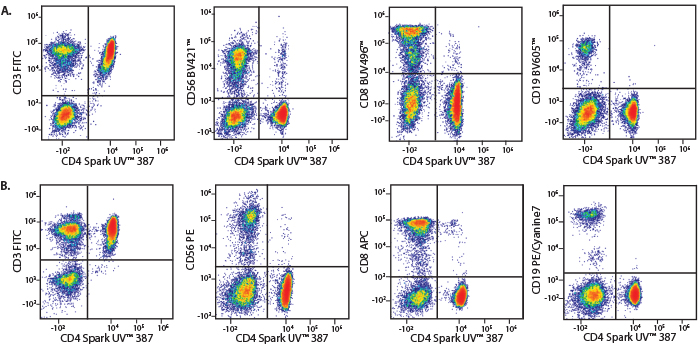
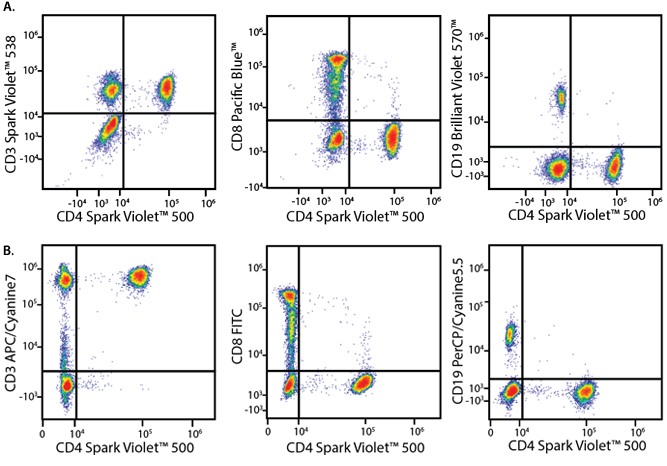
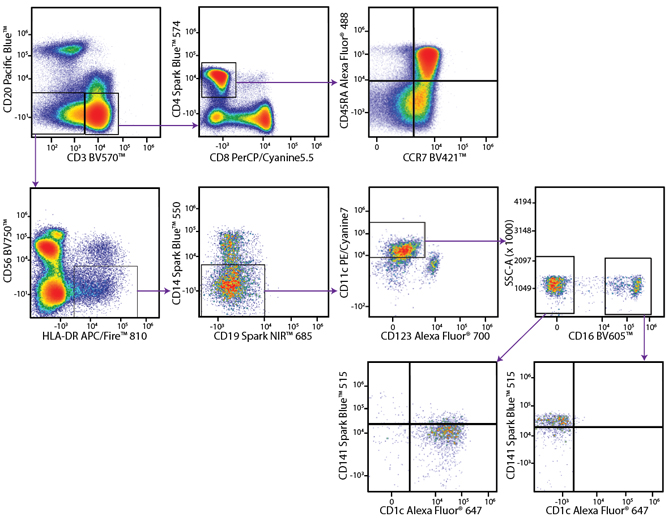
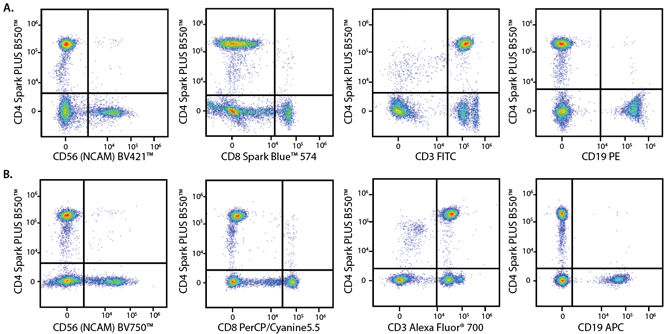
A Perfect Complement to Brilliant Violet™ Dyes
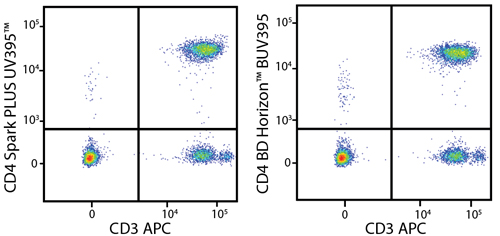
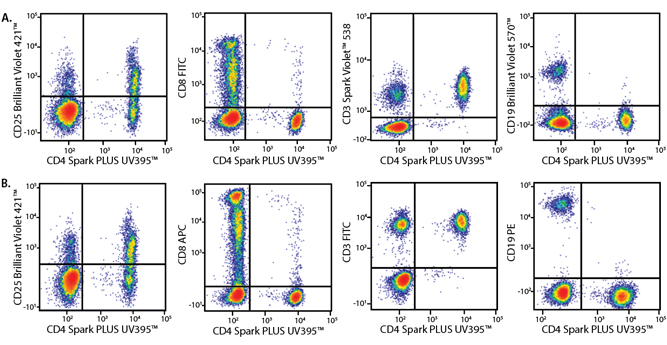
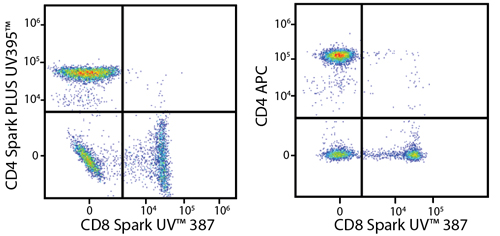
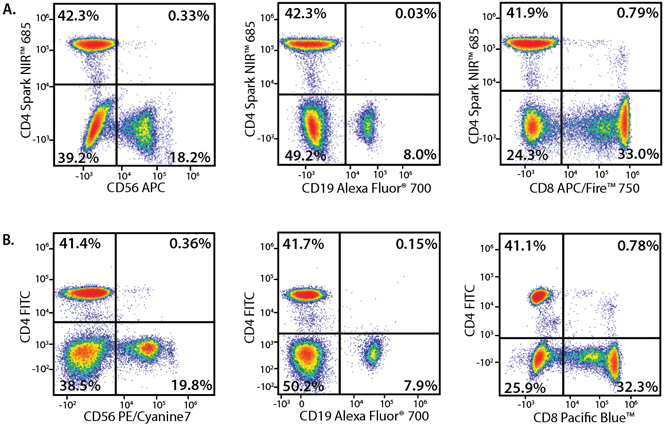
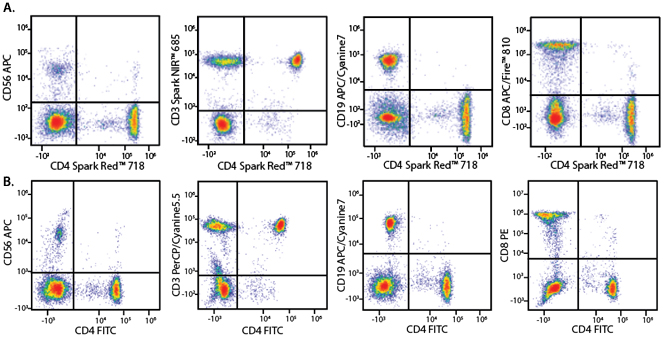
In the data below, Spark Violet™ 538 is utilized with several neighboring Brilliant Violet™ dyes, showcasing its ability to be spectrally unmixed from these dyes in a large, multicolor panel.
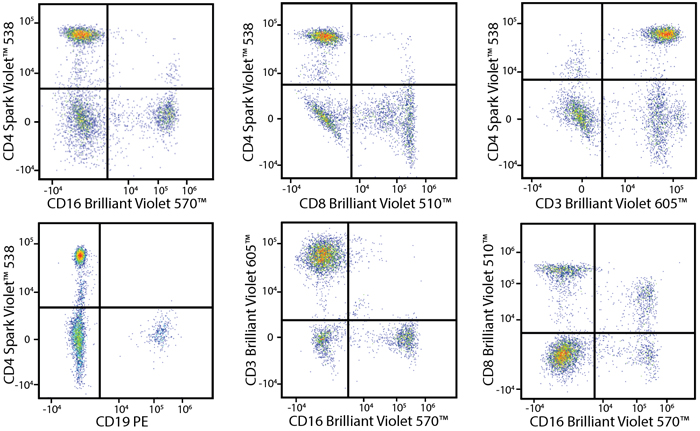
Lysed human whole blood was stained with the indicated antibodies in the presence of Brilliant Stain Buffer. Samples were unmixed on a Cytek™ Aurora Cytometer using compensation beads and cells. All plots are gated on lymphocytes.
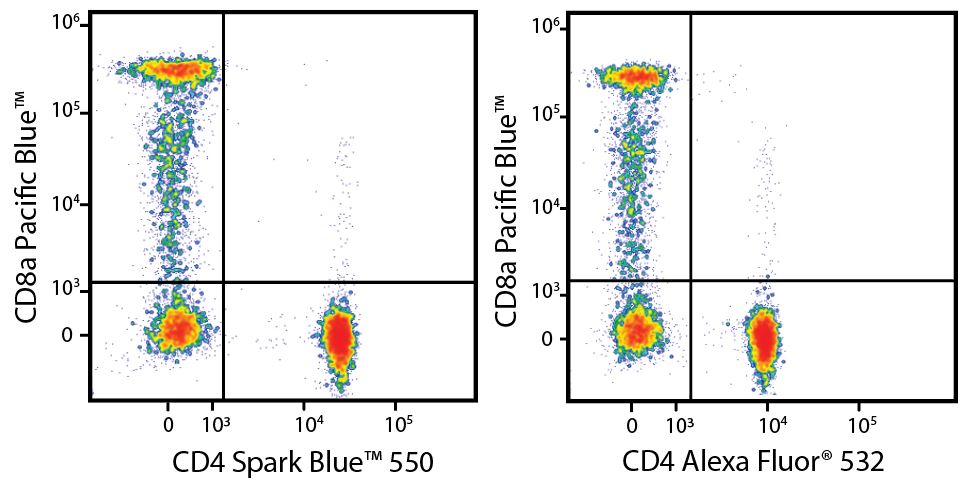
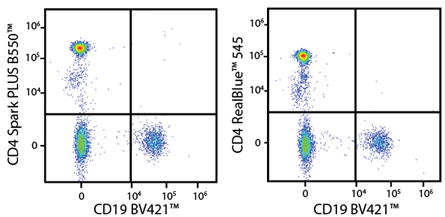
A New Alternative Pacific Orange™
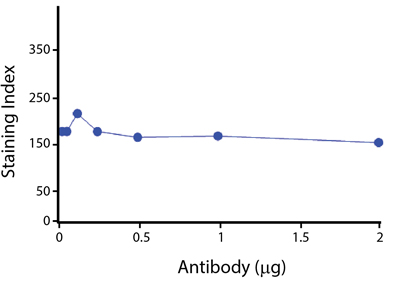
Spark Violet™ 538 is spectrally similar to Pacific Orange™ with a peak emission in the V8 channel on the Cytek™ Aurora. Spark Violet™ 538 offers a brighter signal when compared to Pacific Orange™ in a side-by-side experiment.
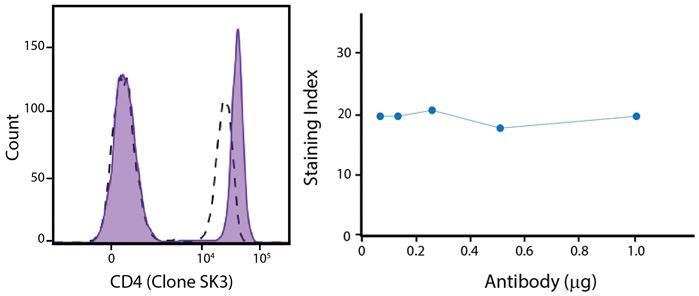
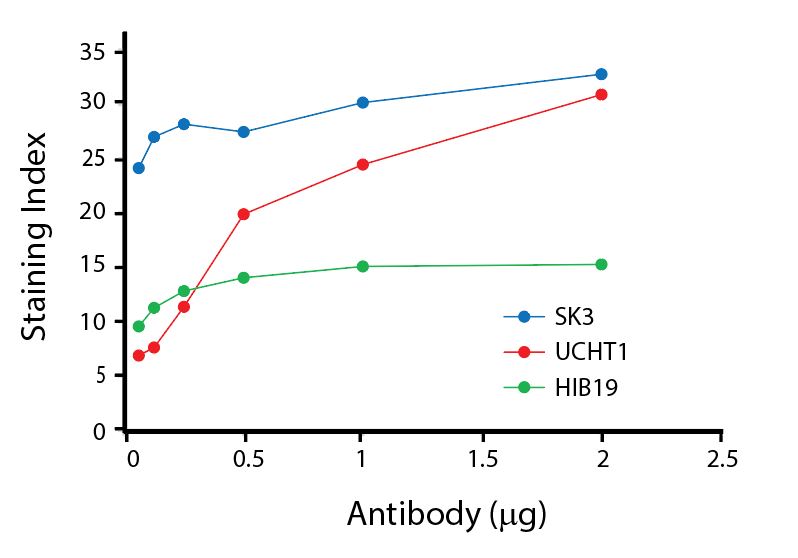
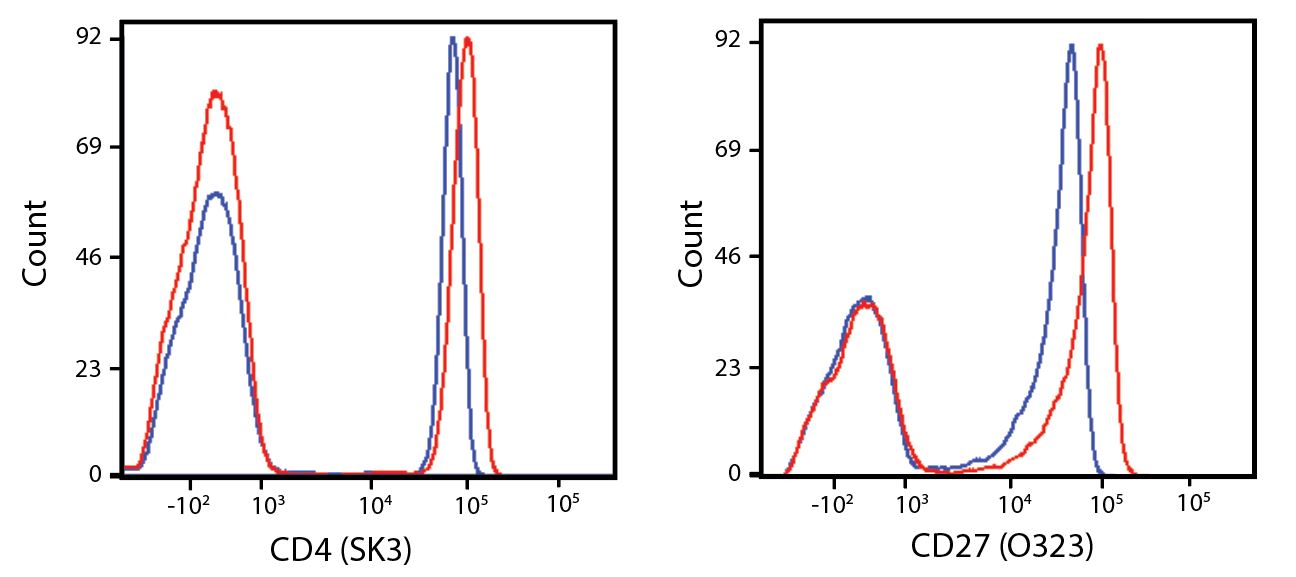
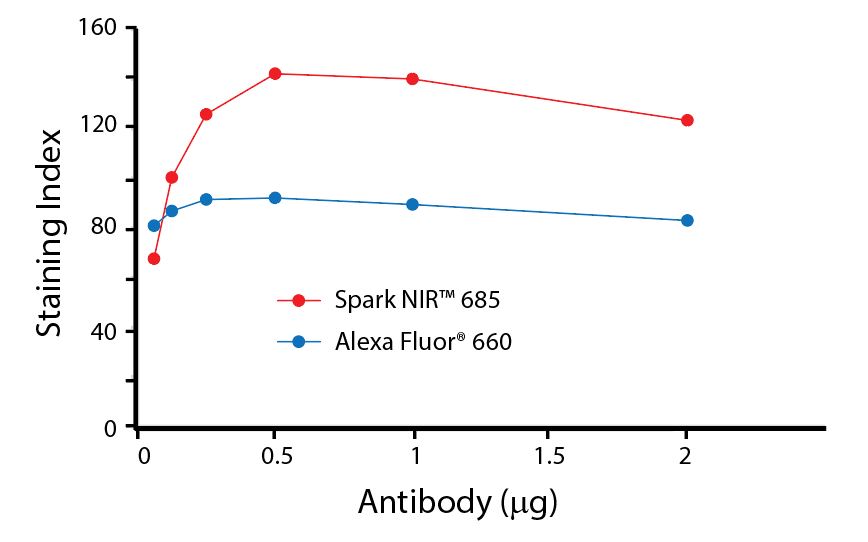
(Left) Human lysed whole blood was stained with anti-human CD4, clone SK3, conjugated to either Spark Violet™ 538 (solid purple) or Pacific Orange™ (clone S3.5, clear dashed) and then analyzed on a Cytek™ Aurora cytometer. Plots are gated on lymphocytes. (Right) Titration curve generated by staining human lysed whole blood with Spark Violet™ 538 conjugated to anti-CD4, clone SK3, antibody.
Stability and Validation Testing
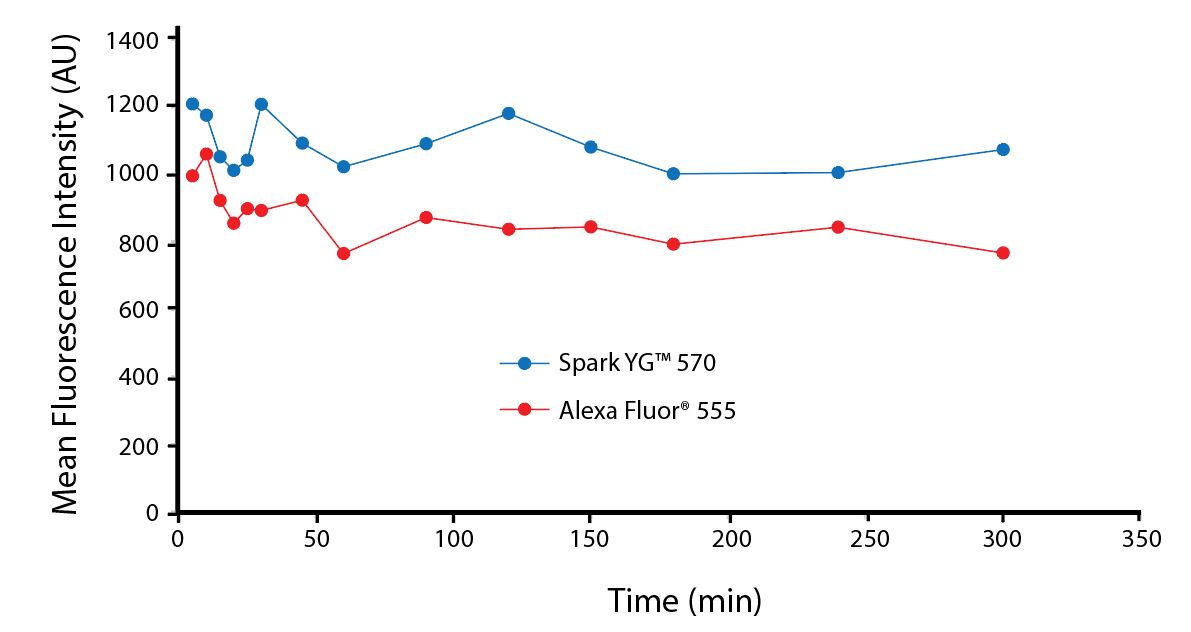
All BioLegend fluorophores undergo rigorous testing procedures to determine how light, heat, and fixation may affect the performance and ensure they will perform reliably. To compare the signal across different conditions and timepoints, we used the Stain Index (formula below) to measure the relative brightness of the antibody
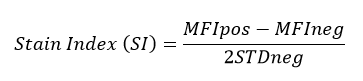
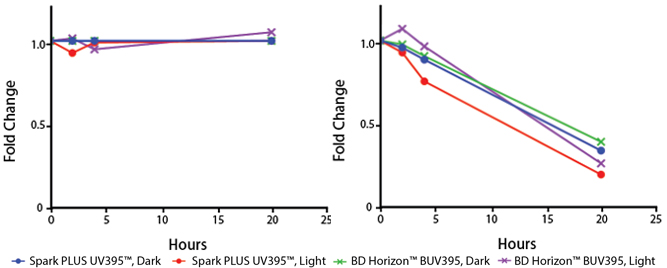
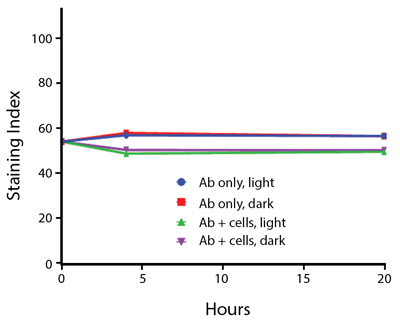
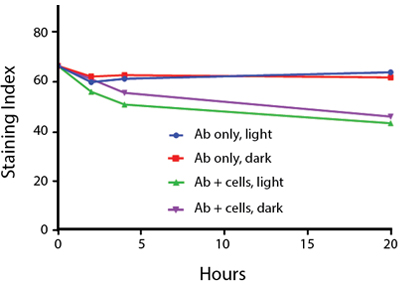
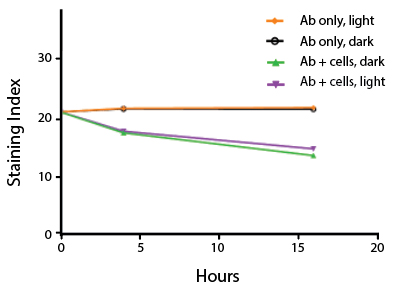
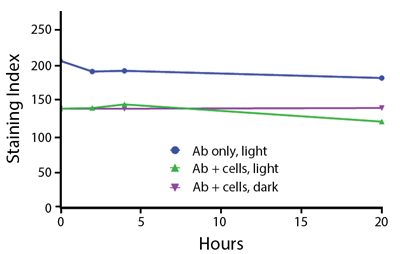
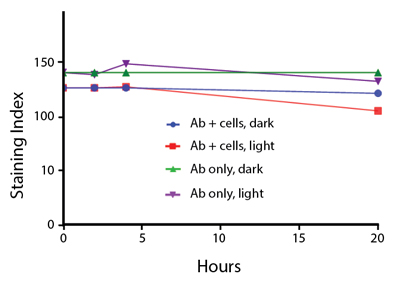
Photostability Testing
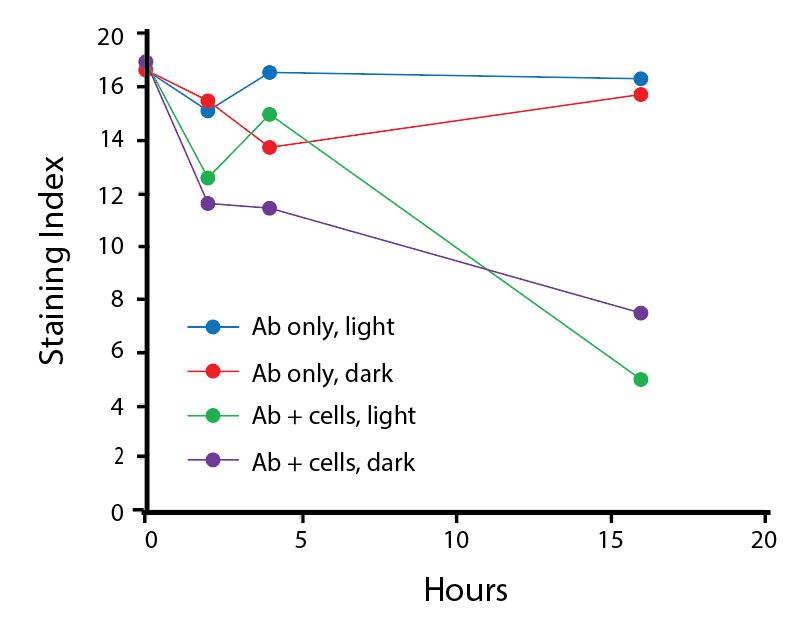
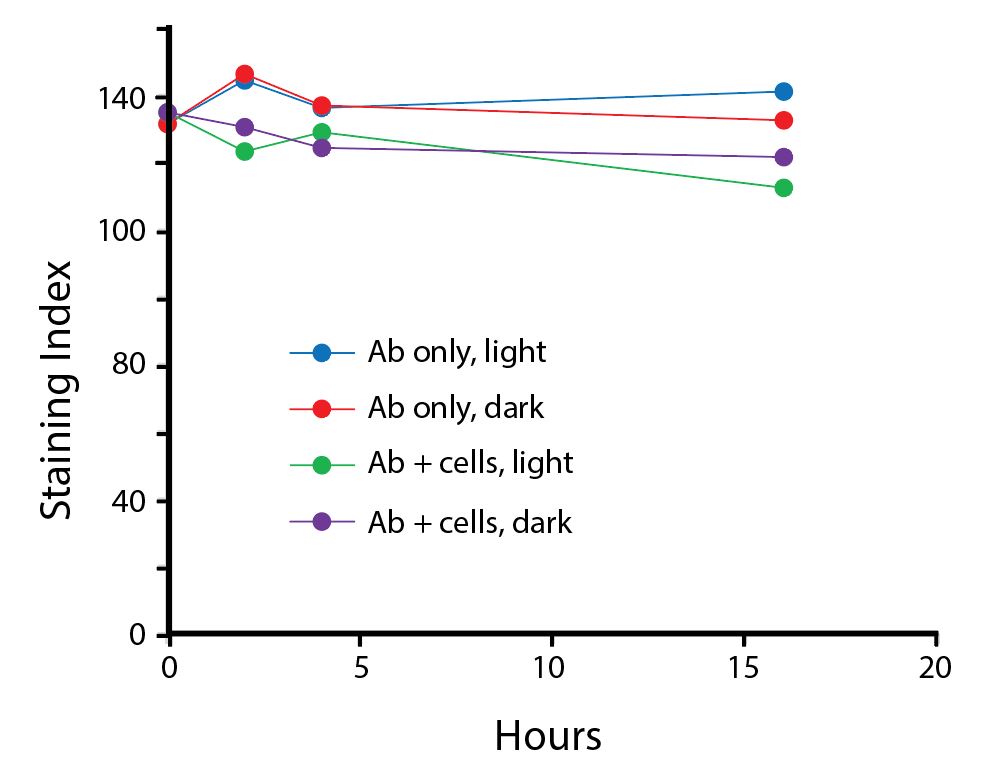
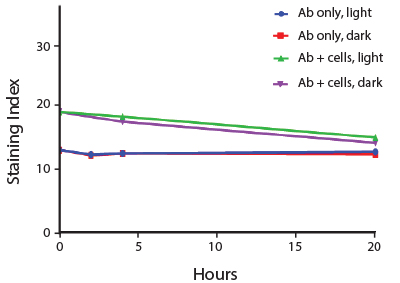
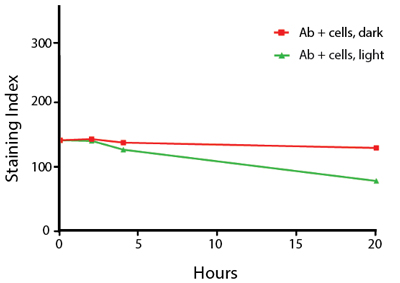
The photostability of Spark Violet™ 538 was tested in two ways that mimic how an antibody may be exposed to light over the course of an experiment.
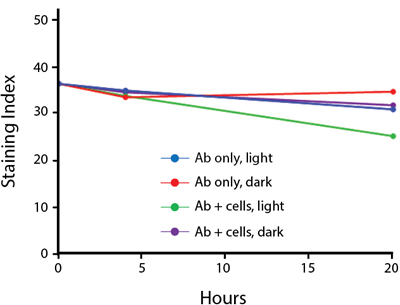
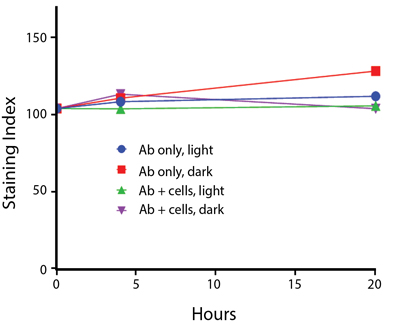
- Antibodies were stored in the dark or exposed to fluorescent lighting. Then, the antibodies were used to stain freshly harvested cell samples and analyzed immediately.
- Cells were stained with antibody that had been kept under recommended storage conditions. Prior to analysis, the stained cells were stored in the dark or exposed to fluorescent lighting.
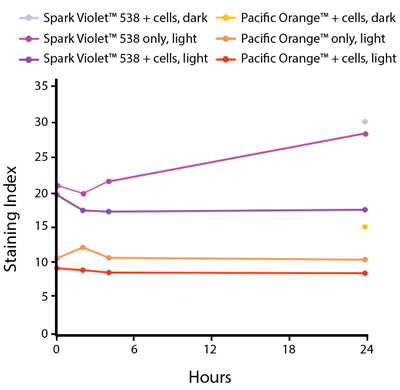
Anti-human CD4 Spark Violet™ 538 (clone SK3) or Pacific Orange™ (clone S3.5) antibodies were stored in a clear vial (dye only) and left exposed to light or protected in the dark, as indicated. Antibodies were stored for the indicated timepoints and then used to stain human lysed whole blood. “Dark” samples were only tested at the 24 hour time point as a control. Samples labeled "dye + cells" contain human lysed whole blood that was stained with the indicated antibody. Stained cells were then left in the light or protected, as indicated.
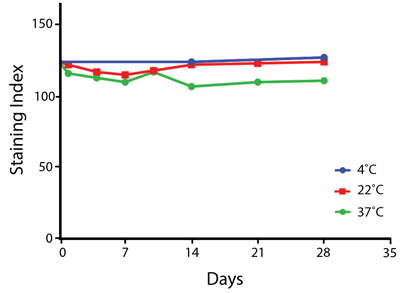
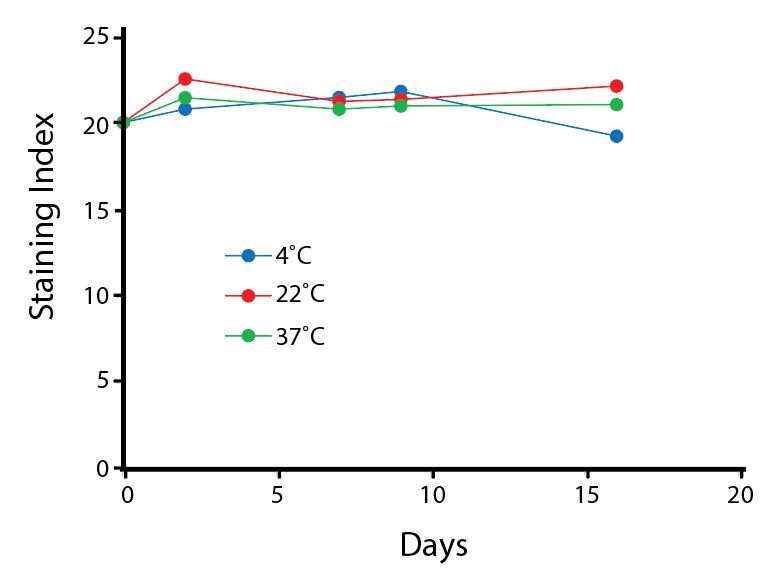
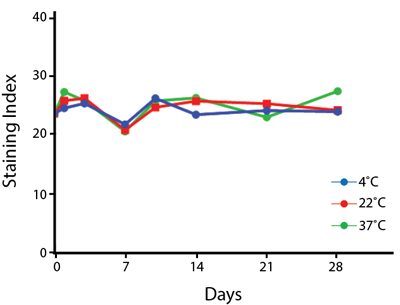
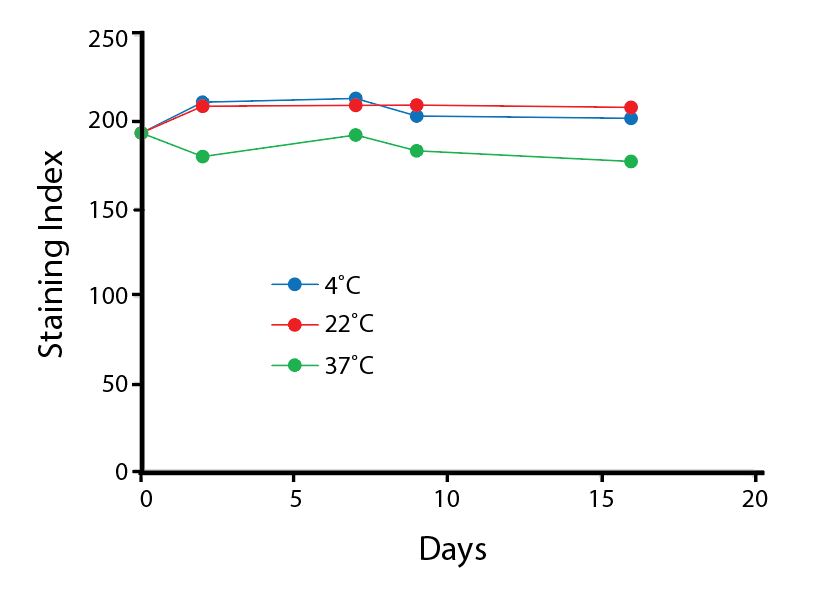
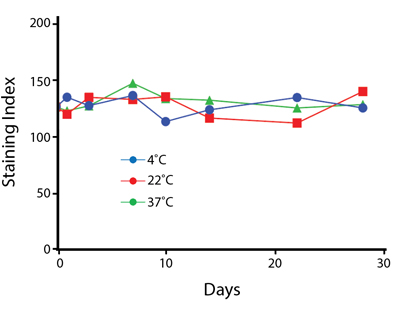
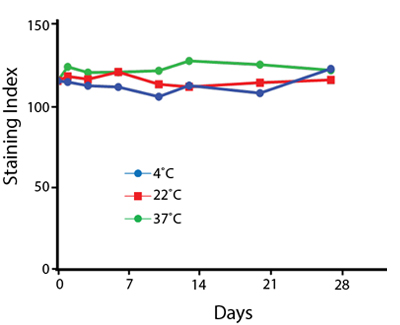
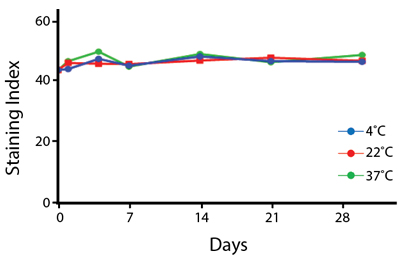
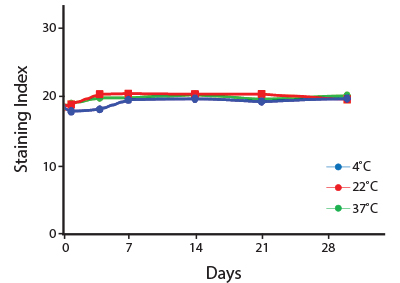
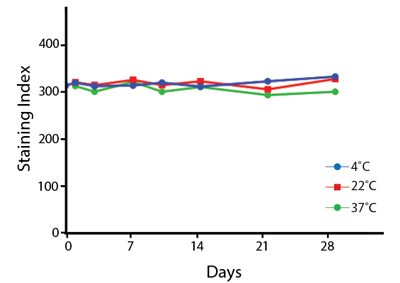
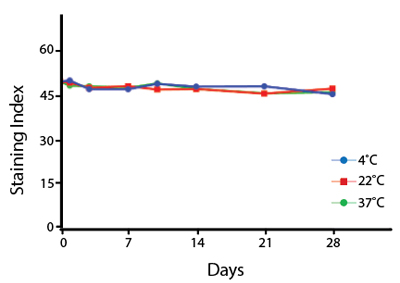
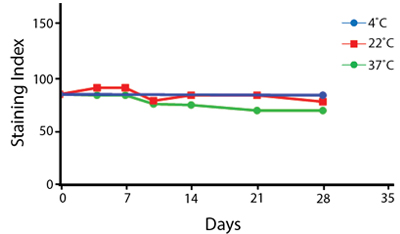
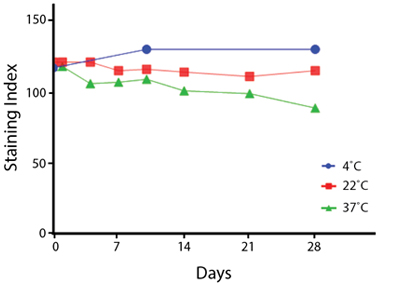
Heat Stability
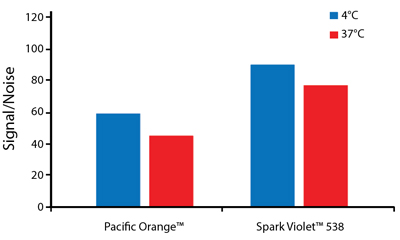
Anti-human CD4 antibodies, clone SK3 Spark Violet™ 538 or clone S3.5 Pacific Orange™, were kept at either 4°C or 37°C for seven weeks. The antibodies were then used to stain human lysed whole blood.
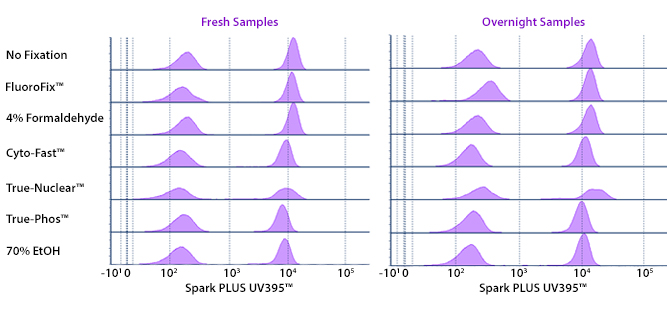
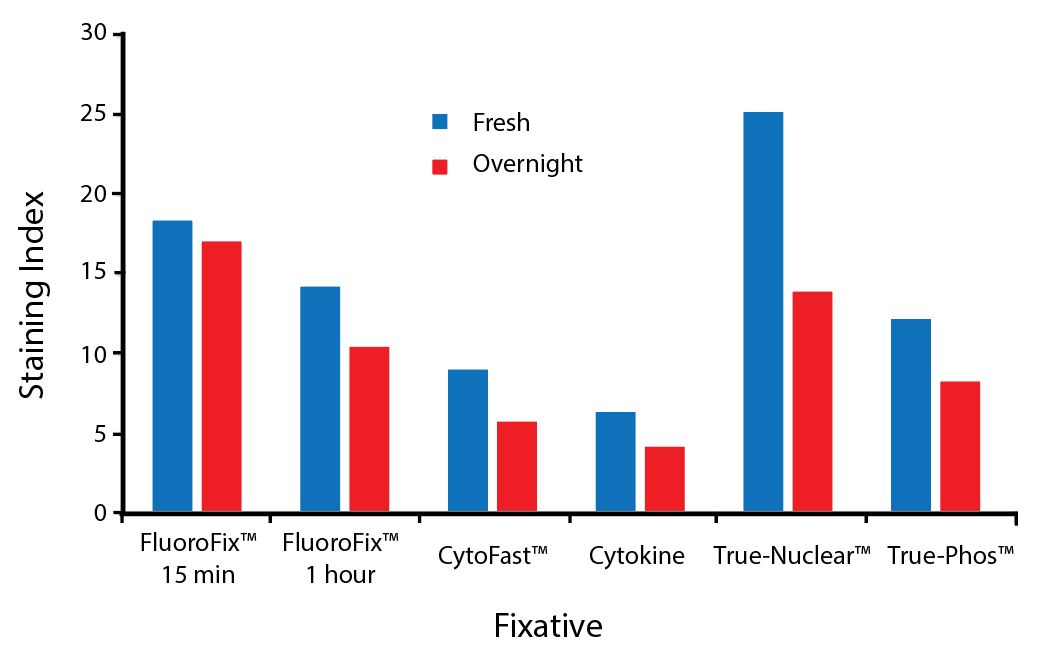
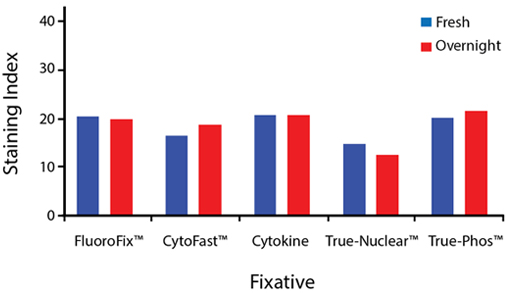
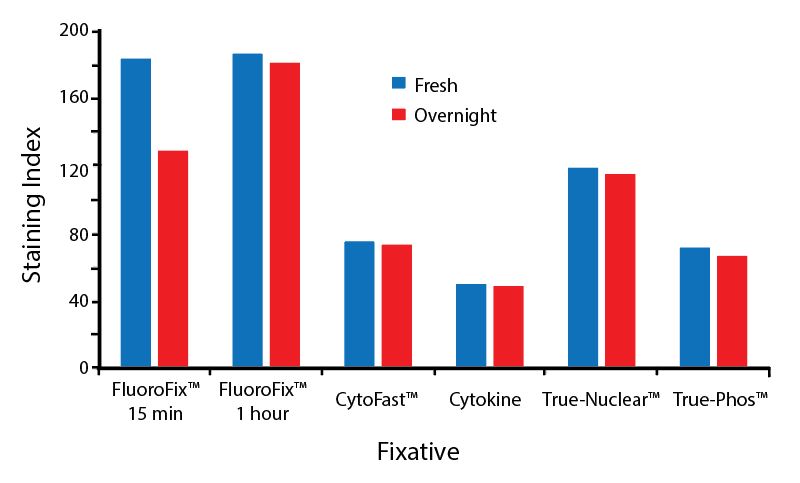
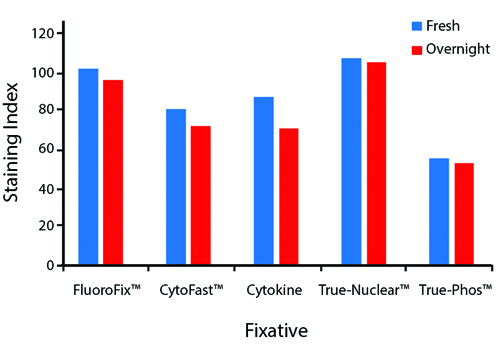
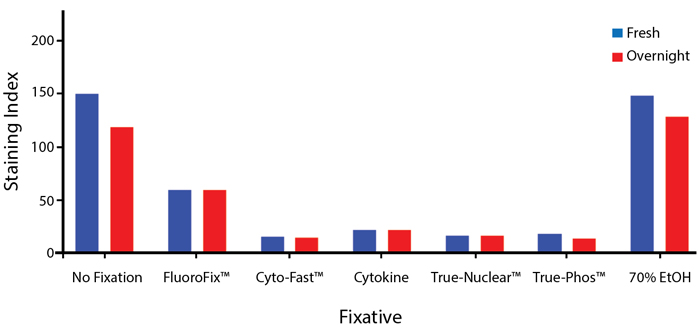
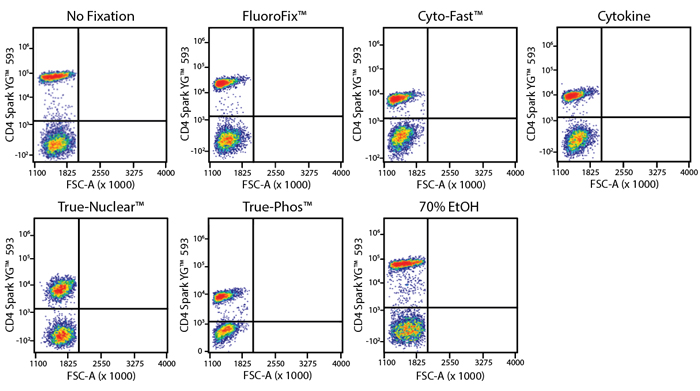
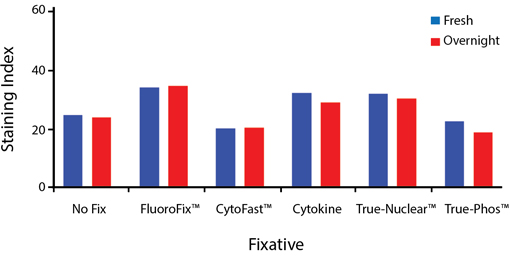
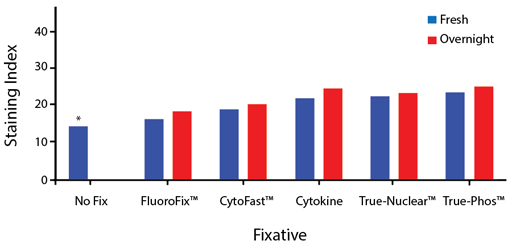
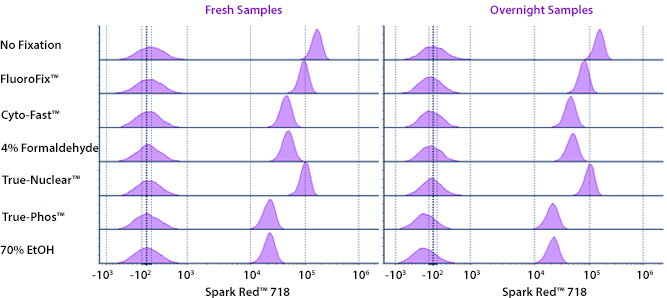
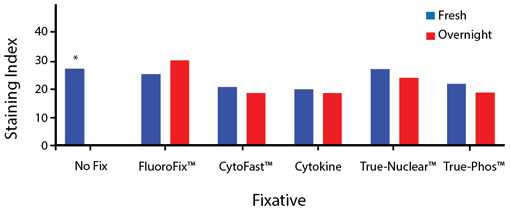
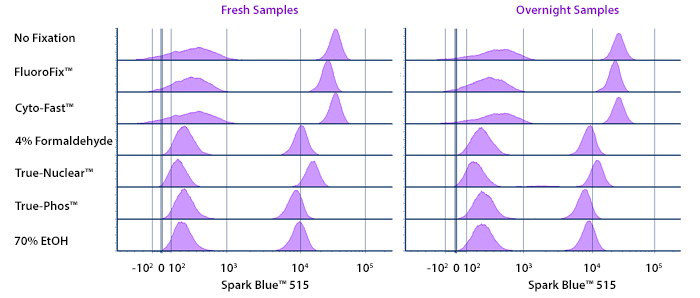
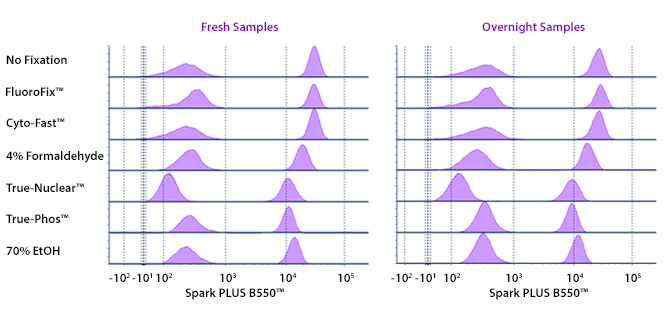
Fixative Stability
Spark Violet™ 538 is compatible with BioLegend buffers, but may be sensitive to alcohol-based buffers. The end user will need to test to ensure their antigen's signal is maintained post-fixation.
A guide to the fixatives used in this experiment:
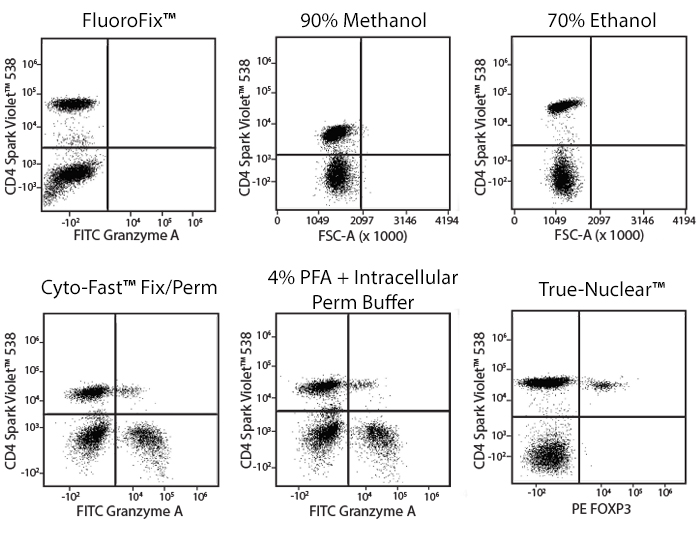
Human PBMCs were stained with anti-human CD4 (clone SK3) conjugated to Spark Violet™ 538 and fixed using the respective protocols for each buffer set. For intracellular staining buffers, additional antibody stains for Granzyme A or FOXP3 were performed. For the alcohols, 90% methanol and 70% ethanol were used. Samples were then transferred into FluoroFix™ Buffer and read on a 5-laser Cytek™ Aurora Cytometer immediately.
 Login/Register
Login/Register 



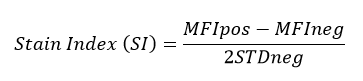











Follow Us