
Multiplex Samples and Hashtags
Multiplex Samples with Hashtag Antibodies
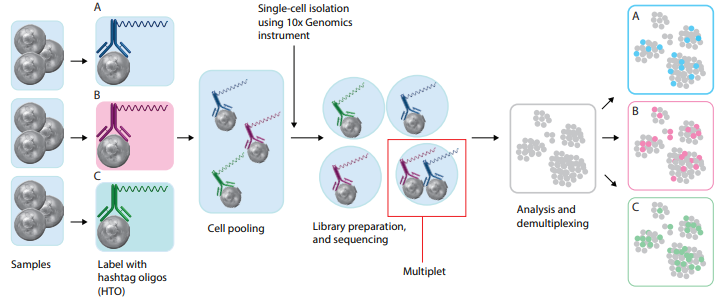
Cell Hashing
To pool multiple samples prior to loading them onto a platform capable of single-cell isolation, try one of our hashtag reagents. Each pre-mixed, ready-to-use hashtag reagent contains a pool of antibodies designed to recognize ubiquitously expressed cell surface markers and is conjugated to a unique barcode. For human samples, our hashtag reagents recognize CD298 and β2-Microglobulin, and for mouse samples, hashtag antibodies recognize CD45 and H-2 MHC Class I.
Benefits of using cell hashing:
- Robust multiplet identification.
- Minimize variability and reduce batch effects between samples.
- Pool smaller samples to reach minimal cell numbers.
Read more about cell hashing and how they can be used to multiplex samples in the initial paper by Stoeckius M, et al.
Tech Insights: Cell Hashing with TotalSeq™ Reagents
Multiplexing samples in single-cell experiments improves sample throughout and provides cost savings. Learn from Nathan Lucas, PhD, as he describes the benefits and technical considerations for our TotalSeq™ hashtag reagents.
Nuclei Hashing
In addition to single-cell analysis in whole and intact cells, single-nucleus analysis makes it possible to characterize cellular states and physiology in tissues that are challenging to dissociate. This includes tissues that are rich in certain cell types like neurons, adipocytes and muscle cells. Single-nucleus analysis can also help when tissue storage is required, as it is difficult to recover and obtain single-cell suspensions from archived frozen material.
To pool isolated nuclei from different samples, we developed nuclear hashing reagents. Our nuclear hashtag antibodies recognize a family of nuclear pore complex proteins and can cross-react with vertebrate organisms and other invertebrate species, such as Xenopus and yeast.
Read more about single nucleus hashing analysis in a Nature Communications paper first describing the technology by Gaublomme, et al.
Understand the Differences between Hashtag Formats
We offer hashtag reagents in our TotalSeq™-A, B, and –C formats. Hashtag reagents from all formats will function similarly, but the workflow and required primers differ.
For TotalSeq™-A reagents, the PCR handle between antibodies and hashtag reagents are different. In practice, this means that you will generate two separate libraries– one for your cell surface markers (referred to as ADTs) and one for hashtag-derived oligos (referred to as HTOs). This is beneficial because the two libraries can be mixed at different ratios prior to sequencing to avoid an excess of reads from the hashtags.
For TotalSeq™-B and –C reagents, the PCR handle is the same between both your antibodies of interest and the hashtag antibodies. In practice, this means that the hashtag and antibody libraries must be prepared together. To avoid an excess of HTO readings, we recommend titrating down the hashtag reagents. For more information on how to titrate TotalSeq™ products, read our FAQs.
 Login/Register
Login/Register 






Follow Us