
How Western Blotting Works
Western Blotting Introduction
Western blotting refers to a routine technique used to separate and identify proteins from complex mixtures. Proteins are separated (potentially under denaturing conditions) in a gel by size before being transferred to a membrane. The protein of interest is probed with specific antibodies and detected through a number of means, commonly chemiluminescence.
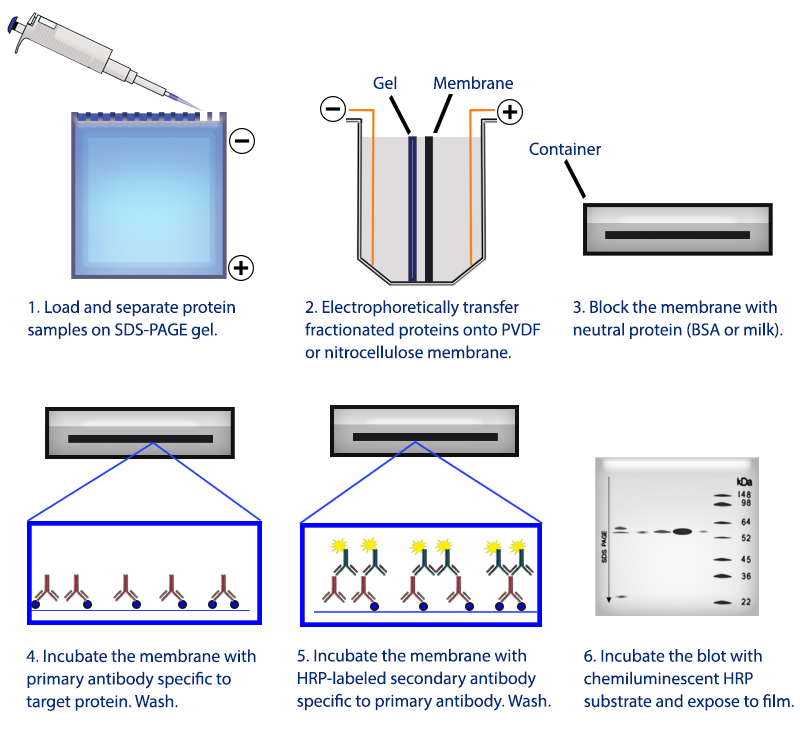
Figure 1. Overview of the western blotting procedure.
Sample Preparation Guide
For western blotting, sample preparation is a key step in the western blotting process. Proper sample treatment ensures you are able to detect proteins of interest. Below, you will find the general methodology used at BioLegend. You may wish to consult literature for more specific protocols relating to your samples of interest.
For cell culture samples
For tissue samples
To start a western blotting procedure, gel electrophoresis is used to separate macromolecules in a sample. The most common type of electrophoresis used is called SDS-PAGE (SDS-Polyacrylamide Gel Electrophoresis). SDS is a type of detergent that adds a negative charge to amino acids in a protein, this along with heat applied during sample prep disrupts the tertiary and secondary structure of the protein. Because of the SDS, all proteins will have the same negative charge, resulting in separation being based on size rather than charge.
Once an electrical field is applied to the gel, small protein molecules move quickly the through the gel matrix toward the positive electrode, while larger proteins move through more slowly, resulting in a series of bands containing proteins of a particular size (Figure 2).
Protein samples are run along side a protein ladder containing several standards of known molecular weights. By using a ladder, the size of proteins in the sample lanes can easily be determined. BioLegend offers the Prime-Step™ Prestained Broad Range Protein Ladder, a three-color protein standard with 10 pre-stained chromophore-conjugated recombinant proteins covering a wide range of molecular weights, from 6.5 to 270 kDa.
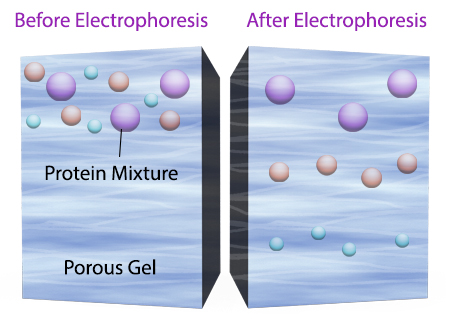
Figure 2. Negatively charged small protein molecules move through the gel matrix, toward the positive electrode, more quickly than larger negatively charged protein molecules.
After gel electrophoresis separated proteins on the gel are transferred onto a nitrocellulose or polyvinylidene difluoride (PVDF) membrane, again utilizing electrophoresis (Figure 3). The membrane is then blocked with neutral proteins, such as BSA or milk, to prevent non-specific binding of antibodies to the surface of the membrane.
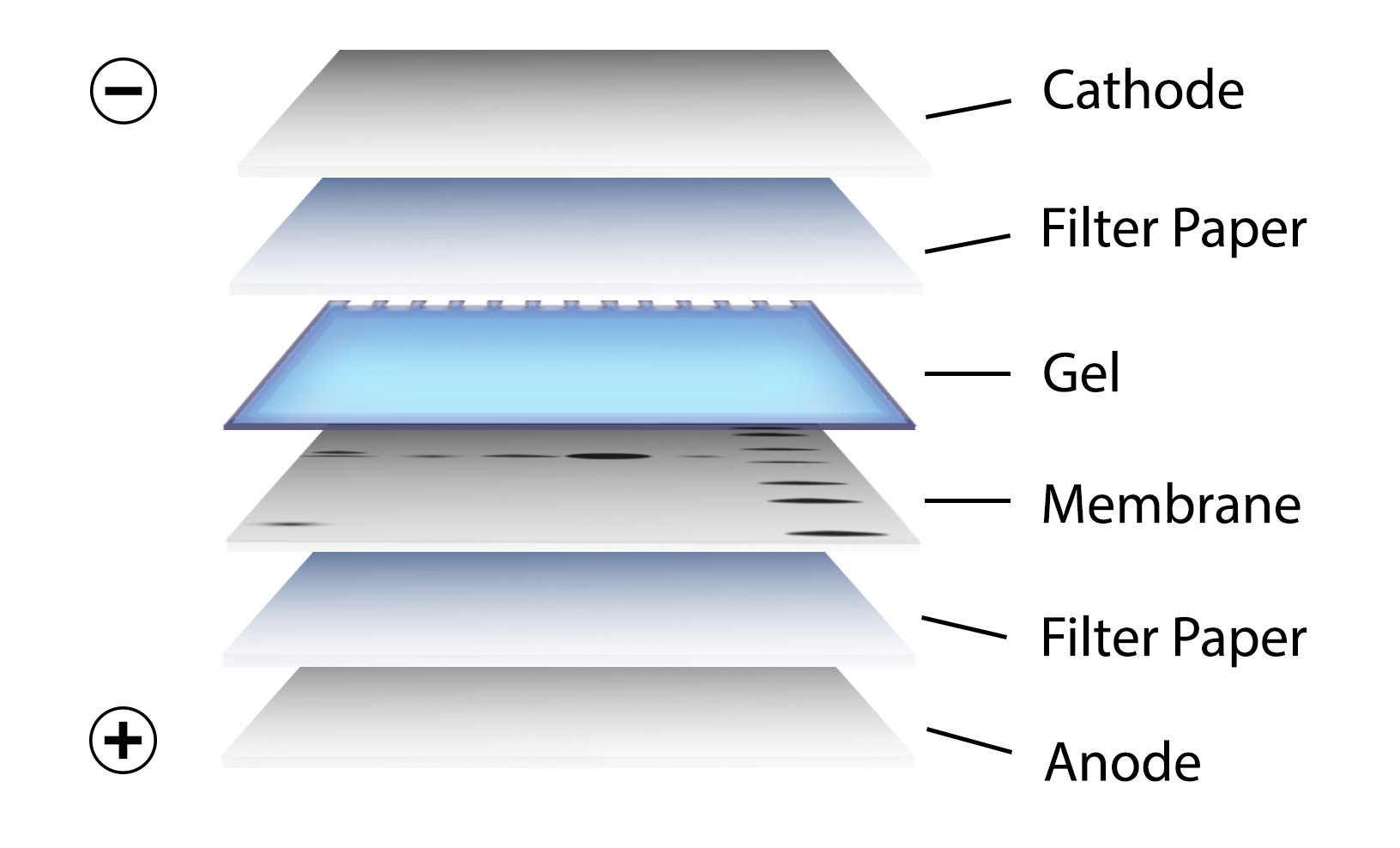
Figure 3. Transfer of the protein bands resulting from SDS-PAGE on to a membrane by electrophoresis
Antibody Binding/Target Detection
It is important to recall that SDS treatment of samples denatures proteins, causing them to lose their native conformation. This is why conformation epitope-specific antibodies and even flow cytometry antibodies may not always work in a western blotting assay. After transfer, the membrane is incubated with primary antibodies that bind specifically to the target protein, the primary antibody is not typically directly detectable. In colorimetric and chemiluminescent detection methods, the membrane is subsequently incubated with a detectable tagged secondary antibody specific to the host species of the primary antibody. Enzyme reporters like alkaline phosphatase (ALP) and horseradish peroxidase (HRP) are then used to generate signal in combination with a substrate like our Western-Ready™ ECL Substrate Kit. Direct-Blot™ products allow users to utilize primary antibodies directly conjugated to HRP, avoiding the need for a secondary reagent and streamlining the western blot process. For fluorescence-based detection methods, fluorophore-conjugated primary antibodies are used. These fluors typically emit in the near infrared range for detection.
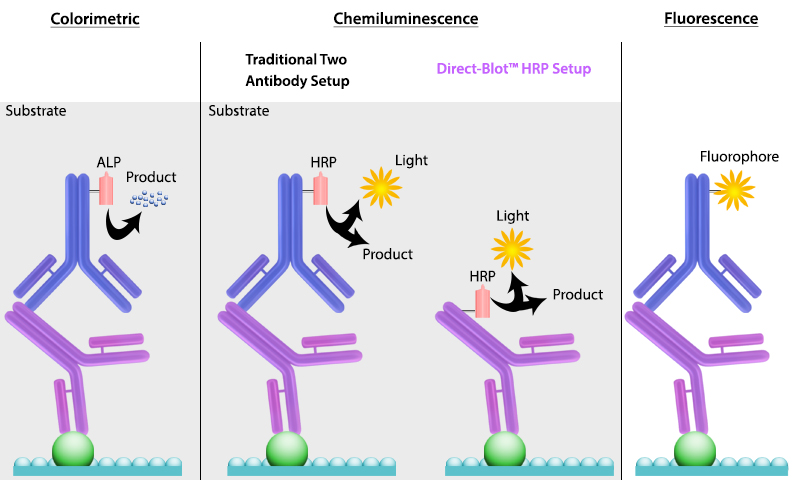
Figure 4. There are multiple ways to detect proteins of interest in a western blot. The colorimetric method detects signal via colored precipitate. In a chemluminescence-based method, light is emitted by the reaction shown. And finally, in fluorescence, a fluorophore-labeled antibody emits the signal.
 Login/Register
Login/Register 






Follow Us