- Other Names
- Chemically-Defined (CD), Serum-free Human Serum Replacement
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
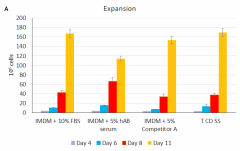
Cell-Vive™ T cell CD Serum Substitute (T CD SS) supports high PBMC-derived T cell expansion and preserves viability and CD4/CD8 balance at similar levels than hAB serum or an equivalent commercially available product (Competitor A). PBMC-derived T cells were activated with 1 µg/mL of Ultra-LEAF™ anti-human CD3 antibody ( Cat. No. 317347), 1 µg/mL of Ultra-LEAF™ anti-human CD28 antibody (302943) in IMDM basal media supplemented with 200 IU/mL recombinant human IL-2 (Cat. No. 791908) and appropriate supplements. Cell expansion (A) and viability (B) were determined on day 4,6, 8 and end of culture (day 11). Flow cytometry was used to determine specific T cell populations, on day 11, by making use of anti-human CD4 (Cat. No. 317408) and anti-human CD8 (Cat. No. 344722) cell markers (C). -

-

| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 420506 | 50 mL | 278€ | ||||
Cell-Vive™ T Cell CD Serum Substitute, GMP is specifically formulated to support PBMC-derived T cell expansion, resulting in equal or higher specific cell numbers compared to the use of human AB serum. It is a Chemically-Defined, serum-free formulation, prepared without human or other animal components, intended to be combined with the basal media of choice, such as IMDM. It is suitable for use in combination with several T cell activation methods and is able to support T cell populations (CD4, CD8) comparable to human AB serum. This GMP product is suggested for use in research and further manufacturing applications. The benefits of this serum substitute include:
- Effective for maintaining CD4/CD8 balance.
- Maintains high cell viability, >90%, throughout cell culture.
- Minimizes safety risks associated with human AB serum.
- Improved lot-to-lot consistency over human AB serum.
- Saves time and costs required to qualify human AB serum
BioLegend Cell-Vive™ GMP cell culture products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include
- Low endotoxin level (< 0.5 EU/mL; After 20x Dilution)
- Mycoplasma and bacterial/fungal growth testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Formulation
- Chemically Defined, serum-free formulation
- Endotoxin Level
- < 0.5 EU/mL (after 20x Dilution)
- Storage & Handling
- Store at -20°C to -5°C. Thaw overnight at 4°C. If necessary product can be kept at 4°C for up to two weeks.
- Application
-
Cell Culture
- Application Notes
-
Complete cell Culture Media Preparation:
Thaw Cell-Vive™ T cell CD Serum Substitute, GMP overnight at 4°C. The product can be stored at 4°C in the dark for up to 2 weeks post-thaw. Do not refreeze after initial thaw. The presence of cloudiness in the product after thaw is normal and relates to the high enrichment of the product. Make sure to mix product prior to use. Once the product is diluted into basal media, no cloudiness should be observed.
Dilute Cell-Vive™ T cell CD Serum Substitute, GMP to 5% v/v in IMDM and 200 IU/mL of recombinant human IL-2 (BioLegend, Cat. No. 791906 or equivalent). Alternatively, the product can be used in combination with your basal media of choice and cytokines of interest. To determine optimal performance, a titration of the product under your desired cell culture setup may be required.
Cell Culture Platform:
Cell-Vive™ T cell CD Serum Substitute, GMP can be used with tissue culture plates, and flasks. If using a bag format, testing is recommended with the specific bag being used.
Cell Activation methods:
Cell-Vive™ T cell CD Serum Substitute, GMP can be used in combination with anti-human CD3 and CD28 antibodies in solution, plate-coated. Optimal values should be determined if using a different culture setup.
For the protocols below, complete media refers to IMDM with 5% v/v Cell-Vive™ T cell CD Serum Substitute, GMP, and 200 IU/mL recombinant human IL-2 (BioLegend, Cat. No. 791906 or equivalent) for T cell culture.
1. Coating plates with anti-human CD3 and CD28 antibodies
Add 1 µg/mL of anti-human CD3 (OKT3 clone, BioLegend, Cat. No. 317326 or equivalent) and 1 µg/mL of anti-CD28 antibodies (CD28.2 clone, BioLegend, Cat. No. 302934 or equivalent) to PBS. Add solution to the vessel, at recommended volume (table below). Incubate for 2 hrs at 37°C in a tissue culture incubator (or overnight at 4°C, preventing evaporation with Parafilm®). Aspirate solution and wash twice with 1 volume of PBS. Wells are ready to be used. Do not let wells dry.
TABLE 1: Recommended volumes of coating solution composed of anti-human CD3 and CD28 antibodies.Vessel
Recommended volume
12- well plate
0.75 mL
6- well plate
2 mL
T25 flask
5 mL
T75 flask
15 mL
2. Soluble antibodies
Add 10 µg/mL of anti-human CD3 (OKT3 clone, BioLegend, Cat. No. 317326 or equivalent) and 5 µg/mL of anti-CD28 antibodies (CD28.2 clone, BioLegend, Cat. No. 302934 or equivalent) to complete media.
PBMC-Derived T cell culture protocol:- Equilibrate appropriate amount of complete media at 37°C for 10-15 min.
- Plate PBMC (fresh or frozen) at 1 x 106 cells/mL and incubate at 37°C and 5% CO2.
- Allow cells to get activated for 3-4 days.
- At day 3-4, dissociate cell clumps by pipetting until a single cell solution is observed.
- Determine cell density.
- Dilute cell culture to 0.25 - 0.5 x 106 cells/mL and incubate at 37°C and 5% CO2.
- Repeat steps 4-6 every 2 days.
- Monitor cell culture to make sure confluency is not reached. Pending donor-to-donor variability exponential expansion may require more frequent feeding. Complete media has been shown to support up to 4 x 106 cells/mL without impact on cell viability and quality.
- Follow with further processing and/or cell analysis.
Parafilm is a registered trademark of Bemis Company, Inc. - Disclaimer
-
BioLegend Cell-Vive™ GMP Cell Culture products are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products
Antigen Details
- Gene ID
- NA

 Login / Register
Login / Register 
















Follow Us