| DNA Dye |
Equivalent Channel/Fluor |
Cell Permeable? |
Binding Mechanism |
| Helix NP™ Blue |
Sytox® Blue, Coumarin, BV421™ |
No |
Single intercalator |
| Helix NP™ Green |
Sytox® Green, FITC |
No |
Bis-intercalator |
| Helix NP™ NIR |
To-Pro-3®, Alexa Fluor® 647, APC |
No |
Single intercalator |
| CytoPhase™ Violet |
Hoechst 33258, Coumarin, BV421™ |
Yes |
Minor groove |
| DRAQ5™ |
Alexa Fluor® 647, APC |
Yes |
Single intercalator |
| DRAQ7 |
Alexa Fluor® 700, APC/Cyanine7 |
No |
Single intercalator |
| 7-AAD |
PE/Cyanine5, PE/Dazzle™ 594 |
No |
Single intercalator |
| DAPI |
Coumarin, BV421™ |
Semi |
Minor groove |
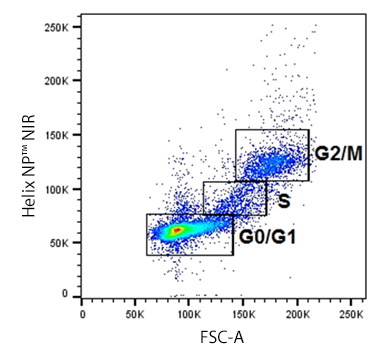
| Permeability is a simple enough concept: can the dye get into the cell to bind DNA if the membrane is intact? Permeability will define the type of assay your dye is useful for. If it's cell-permeant, it's less suitable for a viability assay since it will get into every cell regardless of its health status. Instead, it will work better to give you an idea of a total cell count or cell cycle status. If a dye is not cell-permeant, then it can be helpful in assessing viability. For our Helix NP™ dyes, NP indicates they're "Not cell-Permeant" so they're helpful assessors of cell health. Similarly, DRAQ7™ and Propidium Iodide are also excluded by healthy cells with intact membranes. Other dyes like DAPI and 7-AAD are classically considered not to be cell-permeant. However, they are actually semi-permeant, meaning at high concentrations, they'll get through a live cell's membrane and stain, but to a lesser degree than a dead cell would. |
Mechanisms of Action
|
|
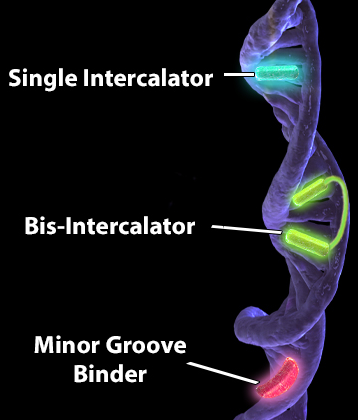
It can also be helpful to understand how these dyes are binding to DNA. Different interactions with DNA have different affinities. The higher the affinity, the tighter the binding and the more easily you can resolve DNA and see more than just a haze around the nucleus. We'll be focusing on dyes that bind the minor groove and intercalators. The minor groove is the narrower of the two grooves formed by the double helix structure of DNA. Minor groove binders generally have a lower affinity than intercalating dyes. Minor groove binders may be a little less flexible as they must follow the groove as it twists around the axis, coming into contact with the edges of base pairs. Binding typically occurs through non-covalent means (i.e., hydrogen bonding of the probe to base pairs).
|
| DNA intercalators actually insert or "intercalate" non-covalently between two sets of adjacent base pairs, causing them to separate and create a pocket for the dye. A part of dye's structure, such as a hydrophobic aromatic ring that bears resemblance to a ring of bases in DNA, can insert itself between sets of base pairs. This can actually cause the DNA to become distorted. Bis-intercalators contain two intercalating moieties connected by a linker that interacts with a groove. As such, they have an even higher affinity than single intercalators and have even been used to image single strands of DNA. Minor groove binders and intercalators can increase in fluorescence due to conformation changes upon binding DNA. It is important to notice that most DNA-binding dyes can be dislodged if the samples are fixed or permeabilized. This is particularly important in scenarios where organic solvents with DNA-denaturing properties are used. For example, methanol is commonly used to help solubilize paraformaldehyde in certain fixation solutions. This can lead to false positives and negatives, making it harder to interpret your data. Alternatively, you can analyze viability with Zombie Dyes, which bind amines on proteins and are more well-suited for fixation and permeabilization conditions. |
 |
|
 Login / Register
Login / Register 







Follow Us