- Clone
- BVD2-23B6 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Granulocyte/macrophage colony-stimulating factor, CSF-α, Pluripoietin-α, Eosinophil colony stimulating factor (Eo-CSF), Burst promoting activity (BPA)
- Isotype
- Rat IgG2a, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
Select size of product is eligible for a 40% discount! Promotion valid until December 31, 2024. Exclusions apply. To view full promotion terms and conditions or to contact your local BioLegend representative to receive a quote, visit our webpage.
Granulocyte/macrophage - colony stimulating factor (GM-CSF) is a hematopoietic factor that is produced by activated T cells, B cells, mast cells, macrophages, fibroblasts and endothelial cells. In addition to supporting colony formation of granulocyte/macrophage progenitors, GM-CSF is a growth factor for erythroid, megakaryocyte and eosinophil progenitors.
Product DetailsProduct Details
- Verified Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Rat
- Immunogen
- E. coli -expressed, recombinant human GM-CSF
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- Less than 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
ELISA Capture - Quality tested
ELISPOT Capture, Neut, IP, WB - Reported in the literature, not verified in house - Recommended Usage
-
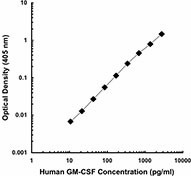
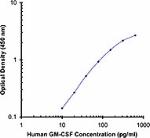
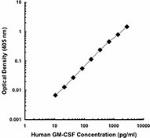
Each lot of this antibody is quality control tested by ELISA assay. For ELISA capture applications, a concentration range of 0.25 - 1.0µg/mL is recommended. To obtain a linear standard curve, serial dilutions of GM-CSF recombinant protein ranging from 500 to 4 pg/mL are recommended for each ELISA plate. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
ELISA or ELISPOT Capture1-6: The Purified BVD2-23B6 antibody is useful as the capture antibody in a sandwich ELISA or ELISPOT assay, when used in conjunction with the biotinylated BVD2-21C11 antibody (Cat. No. 502304) as the detecting antibody. The Ultra-LEAF™ Purified antibody is suggested for ELISPOT capture.
Neutralization1-4: The Ultra-LEAF™ Purified antibody (Endotoxin <0.01 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for neutralization of human GM-CSF bioactivity (Cat. Nos. 502205 - 502210).
Additional reported applications (for the relevant formats) include: immunoprecipitation3 and Western blotting.
Note: For testing human GM-CSF in serum or plasma, BioLegend's ELISA Max™ Sets (Cat. Nos. 432001 and 432004) are specially developed and recommended. -
Application References
(PubMed link indicates BioLegend citation) -
- Bacchetta R, et al. 1990. J. Immunol. 144:902.
- Kita H, et al. 1991. J. Exp. Med. 174:745.
- Abrams J, et al. 1992. Immunol. Rev. 127:5.
- Abrams J, et al. 1994. Eosinophils in Allergy and Inflammation. Marcel Dekker New York. p.133.
- Mahanty S, et al. 1992. J. Immunol. 148:3567.
- Klinman D, et al. 1994. Curr. Prot. Immunol.. John Wiley and Sons New York. Unit 6.19.
- RRID
-
AB_2832790 (BioLegend Cat. No. 502210)
AB_2832790 (BioLegend Cat. No. 502208)
AB_2832790 (BioLegend Cat. No. 502209)
AB_2832790 (BioLegend Cat. No. 502205)
AB_2832790 (BioLegend Cat. No. 502206)
AB_2832790 (BioLegend Cat. No. 502207)
Antigen Details
- Structure
- Cytokine; 22 kD (Mammalian)
- Bioactivity
- Growth/development granulocyte/macrophage progenitors; differentiates myeloblasts/monoblasts; synergizes with Epo proliferation of erythroid/megakaryocytic progenitors
- Cell Sources
- T cells, macrophages, fibroblasts, endothelial cells, mast cells
- Cell Targets
- Granulocyte/macrophage/erythroid/megakaryocytic progenitors, myeloblasts, monoblasts
- Receptors
- Heterodimer GM-CSFR α subunit (CDw116); β-subunit (CDw131) which is also shared the IL-3 and IL-5 receptor α chains
- Biology Area
- Cell Biology, Stem Cells
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
1. Fitzgerald K, et al. Eds. 2001. The Cytokine FactsBook. Academic Press San Diego.
2. Demetri G, et al. 1991. Blood 78:2791.
3. Fan D, et al. 1991. In vivo 5:571.
4. Negrin R, et al. 1992. Adv. Pharmacol. 23:263. - Regulation
- Synergistic with IL-1, IL-3, G-CSF; E21R competitive antagonist for receptor binding; stored in ECM with heparan sulfate proteoglycans
- Gene ID
- 1437 View all products for this Gene ID
- Specificity (DOES NOT SHOW ON TDS):
- GM-CSF
- Specificity Alt (DOES NOT SHOW ON TDS):
- GM-CSF
- App Abbreviation (DOES NOT SHOW ON TDS):
- ELISA Capture,ELISPOT Capture,IP,Neut,WB
- UniProt
- View information about GM-CSF on UniProt.org
Related FAQs
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Other Formats
View All GM-CSF Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Purified anti-human GM-CSF | BVD2-23B6 | ELISA Capture,IP,WB |
| Ultra-LEAF™ Purified anti-human GM-CSF | BVD2-23B6 | ELISA Capture,ELISPOT Capture,IP,Neut,WB |
Customers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
 Login / Register
Login / Register 











Follow Us