- Other Names
- Red blood cell lysis buffer, lysis buffer, CD buffer, RBC Buffer
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

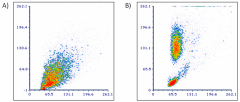
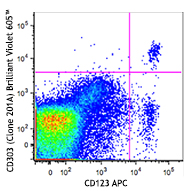
Human peripheral whole blood was analyzed by flow cytometry before the lysis of red blood cells (RBC) (A) or after the treatment with 1X solution of Cell-Vive™ CD RBC Lysis Buffer 10X, GMP (B).
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 420514 | 200 mL | 250 CHF | ||||
Cell-Vive™ CD RBC Lysis Buffer 10X, GMP is a chemically-defined buffer designed to insure optimal lysis of human red blood cells (RBC) with minimal effects on leukocytes. This product is formulated without animal-derived components or preservatives, contains low levels of endotoxin (< 1EU/mL), and is tested negative for mycoplasma and microbial growths. Cell-Vive™ CD RBC lysis Buffer 10X, GMP is a 10X solution that should be diluted to 1X before use. This GMP product is for research and further ex vivo bioprocessing use only.
Product DetailsBioLegend Cell-Vive™ GMP cell culture products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include
- Low endotoxin level (< 1 EU/mL)
- Mycoplasma and bacterial/fungal growth testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Formulation
- Chemically-Defined, no preservatives. Cell-Vive™ CD RBC Lysis Buffer (10X), GMP is a 10X solution that should be diluted to 1X using water
- Endotoxin Level
- < 1 EU/mL
- Preparation
- Chemically-Defined, no preservatives. Cell-Vive™ CD RBC Lysis Buffer (10X), GMP is a 10X solution that should be diluted to 1X using water. Warm the 1X solution to room temperature before use.
- Storage & Handling
- Store between 2°C-8°C
- Recommended Usage
-
Cell-Vive™ CD RBC Lysis Buffer (10X), GMP is a 10X solution that should be diluted to 1X using water. 2 ml of 1X Cell-Vive™ CD RBC Lysis Buffer, GMP is enough to lyse up to 100 μl of whole blood.
- Application Notes
-
Cell-Vive™ CD RBC Lysis Buffer (10X), GMP is recommended for the lysis of human red blood cells. This buffer has a chemically-defined formulation without animal-derived components, it contains low levels of endotoxin (< 1EU/mL), and it is tested negative for mycoplasma and microbial growths. Use Cell-Vive™ CD RBC Lysis Buffer (10X), GMP for highly sensitive assays.
Cell-Vive™ CD RBC Lysis Buffer (10X), GMP is a 10X formulation that contains no preservatives, handle under aseptic conditions as needed.
- Additional Product Notes
-
Human Red Blood Cells lysis protocol
- Dilute the Cell-Vive™ CD RBC Lysis Buffer (10X), GMP to 1X working concentration with deionized water. Warm the 1X solution to room temperature before use.
- Add 2.0 ml of 1X RBC Lysis Buffer to each tube containing up to 100 μl of human whole blood.
- Gently vortex each tube immediately after adding the lysing solution. Incubate at room temperature for 10-15 minutes.
- Centrifuge 350 x g for 5 minutes. Aspirate supernatant without disturbing the pellet.
- Resuspend the pellet in the appropriate buffer (e.g., BioLegend Cell-Vive™ CD Cell Staining Buffer, GMP Cat. No. 420508), and wash 1X.
- Resuspend and proceed with further procedures.
- Disclaimer
-
BioLegend Cell-Vive™ GMP Cell Culture products are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Antigen Details
- Gene ID
- NA
 Login / Register
Login / Register 














Follow Us