- Other Names
- Colony stimulating factor 2, CSF2, Granulocyte/macrophage colony-stimulating factor, CSF-α, Pluripoietin-α, Eosinophil colony stimulating factor (Eo-CSF), Burst promoting activity (BPA)
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
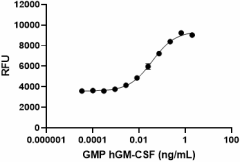
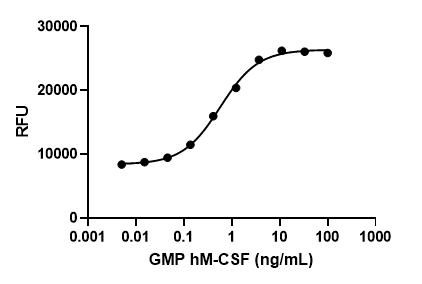
GMP recombinant human GM-CSF induces dose-dependent proliferation of TF-1 erythroleukemic cells. Deep Blue Cell Viability™ Kit (Cat. No. 424701) is used to measure the proliferation. The ED50 range for this effect is 0.01 – 0.04 ng/mL.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 572916 | 100 µg | 1231 CHF | ||||
| 572914 | 25 µg | 382 CHF | ||||
GM-CSF plays a key role in signaling emergency hemopoiesis (predominantly myelopoiesis) in response to infection, including the production of granulocytes and macrophages in the bone marrow and their maintenance, survival, and functional activation at sites of injury or insult. The receptor for GM-CSF is a heterodimer that comprises a major binding subunit (GMRα) and a major signaling subunit (βc). The receptor subunits are always co-expressed on the surface of leukocytes, with βc being expressed at lower levels than GMRα. Certain non-hemopoietic cell types have also been reported to express the GM-CSF receptor and to respond to GM-CSF stimulation in vitro, although the in vivo significance of these observations remains uncertain.
Product DetailsBioLegend Cell-Vive™ GMP Recombinant proteins are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include:
- Low endotoxin level (≤ 0.1 EU/μg)
- Purity (≥ 95% or higher)
- Bioburden testing
- Mycoplasma testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- Quality audits per ISO 13485:2016
- QA review of released products
Product Details
- Source
- Human GM-CSF, amino acids Ala18- Glu144 (Accession# NM_000758) was expressed in E. coli.
- Molecular Mass
- The 127 amino acid N-terminal methionylated recombinant protein has a predicted molecular mass of 14.5 kD. The DTT-reduced protein migrates at approximately 14 kD and the non-reduced protein migrates with slightly greater mobility by SDS-PAGE.
-
N-terminal
Sequence Analysis - Ala-Pro-Ala-Arg-Ser-Pro-Ser-Pro-Ser-Thr
- Purity
- ≥ 95%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.1 µm filtered protein solution is in PBS, pH 7.2.
- Endotoxin Level
- Less than or equal to 0.1 EU per μg protein as determined by the LAL method
- Residual Host Cell Protein Content
- ≤ 0.500 ng/μg by ELISA
- Concentration
- 500 µg/mL
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% endotoxin-free BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week or stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 0.01 – 0.04 ng/mL as determined by the dose-dependent stimulation of TF-1 cell proliferation. Deep Blue Cell Viability™ Kit (Cat. No. 424701) is used to measure the proliferation. The specific activity of Cell-Vive™ GMP Recombinant Human GM-CSF (carrier-free) is ≥ 1.8 x 107 IU/mg when compared against the WHO International Standard for human GM-CSF (NIBSC code: 88/646).
- Application
-
Bioassay
Cell Culture - Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are validated in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
- Product Citations
-
- Disclaimer
-
BioLegend Cell-Vive™ GMP Recombinant proteins are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Antigen Details
- Structure
- Cytokine
- Distribution
-
T cells, macrophages, fibroblasts, endothelial cells, mast cells.
- Function
- Synergistic with IL-1, IL-3, G-CSF; E21R competitive antagonist for receptor binding; stored in ECM with heparan sulfate proteoglycans
- Interaction
- Granulocyte/macrophage/erythroid/megakaryocytic progenitors, myeloblasts, monoblasts, dendritic cells, T cells.
- Ligand/Receptor
- Heterodimer GM-CSFR α subunit (CDw116); β-subunit (CDw131) in common
- Bioactivity
- Measured by its ability to induce proliferation of TF-1 erythroleukemic cells.
- Cell Type
- Embryonic Stem Cells, Hematopoietic stem and progenitors
- Biology Area
- Cell Biology, Stem Cells
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
- Hercus TR, et al. 2009. Blood. 114:1289-98.
- Hayashida K, et al. 1990. Proc Natl Acad Sci USA. 87:9655-9.
- Walker F & Burgess AW. 1985. EMBO J. 4:933-9.
- Kitamura T, et al. 1989. J Cell Physiol. 140:323-34.
- Gene ID
- 1437 View all products for this Gene ID
- UniProt
- View information about GM-CSF on UniProt.org
 Login / Register
Login / Register 














Follow Us