- Other Names
- TGFB, DPD1, transforming growth factor, Transforming Growth Factor Beta 1, TGF-Beta-1
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

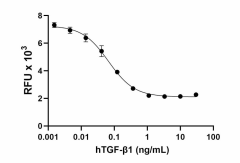
GMP Recombinant human TGF-β1 inhibits the proliferation of mouse HT-2 cells induced by recombinant mouse IL-4 (Cat. No. 574302) in a dose-dependent manner. The ED50 for this effect is 0.05 – 0.25 ng/mL.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 781816 | 100 µg | 1975 CHF | ||||
TGF-β1 is synthesized in the cells as a 390-amino acid precursor. Furin cleaves the protein at residue 278, yielding an N-terminal cleavage product which corresponds to the latency-associated peptide (LAP), and the 25-kD C-terminal portion of the precursor constitutes the mature TGF- β1. TGF-β activators can release TGF-β from LAP. These activators include proteases that degrade LAP, thrombospondin-1, reactive oxygen species, and integrins avb6 and avb8. Mouse TGF-β converts naïve T cells into regulatory T (Treg) cells that prevent autoimmunity. Although human TGF- β1 is widely used for inducing FOXP3+ in vitro, it might not be an essential factor for human Treg differentiation. Th17 murine can be induced from naïve CD4+ T cells by the combination of TGF-β1 and IL-6 or IL-21. Nevertheless, the regulation of human Th17 differentiation is distinct. TGF-β1 seems to have dual effects on human Th17 differentiation in a dose-dependent manner. While TGF-β1 is required for the expression of RORγt, in human naive CD4+ T cells from cord blood, TGF-β1 can inhibit the function of RORγt at high doses. By using serum-free medium, it has been clarified that the optimum conditions for human Th17 differentiation are TGF-β1, IL-1β, and IL-2 in combination with IL-6, IL-21 or IL-23.
Product DetailsBioLegend Cell-Vive™ GMP Recombinant proteins are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include:
- Low endotoxin level (≤0.1 EU/μg)
- Purity (≥ 95% or higher)
- Bioburden testing
- Mycoplasma testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- Quality audits per ISO 13485:2016
- QA review of released products
Product Details
- Source
- Human TGF-β1 amino acid (Ala279-Ser390) (Accession: # P01137), was expressed in CHO cells.
- Molecular Mass
- The 112 amino acid recombinant protein has a predicted molecular mass of approximately 12.7 kD. The DTT-reduced and non-reduced protein migrates at approximately 14 kD and 28 kD respectively by SDS-PAGE. The predicted N-terminal amino acid is Ala.
-
N-terminal
Sequence Analysis - Ala-Leu-Asp-Thr-Asn-Tyr-(Cys)-Phe-Ser-(Ser)
- Purity
- ≥ 95%, as determined by Coomassie stained SDS-PAGE
- Formulation
- Protein was lyophilized from 0.2 μm filtered solution contain 0.1% TFA, 10% Acetonitrile.
- Endotoxin Level
- Less than or equal to 0.1 EU per μg protein as determined by the LAL method
- Residual Host Cell Protein Content
- ≤0.500 ng/μg by ELISA
- Concentration
- 100 μg size is lyophilized
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at - 20°C or colder until the expiration date. Reconstitute lyophilized protein in sterile 4 mM HCL. Before reconstitution, make sure sterile 4 mM HCL acid and product are at room temperature. Quickly spin the vial or gently tap down on the vial to make sure the lyophilized product is at the bottom of the vial before opening. Use aseptic techniques to add the required volume of reconstitution buffer (sterile 4 mM HCL) to the vial, to obtain the recommended stock concentration 250 μg/mL. Close the vial and leave at ambient temperature for 15-20 minutes. Then gently invert the vial several times or until all of the lyophilized product dissolves. Leave the vial at room temperature for another 15 minutes. If small particulates are still observed after 15 minutes, incubate at room temperature for an additional 30 minutes, and leave the vial at 2°C - 8°C overnight. Next day, invert the vial several times or gently pipette the solution up and down before use. If needed, transfer the reconstituted stock solution to a sterile container for additional dilution to no less than 100 μg/mL. Small working aliquots in polypropylene tubes can be made after reconstitution and store the vials at -20°C or lower. Avoid freeze/ thaw cycles. Carrier protein such as 0.2 - 1% endotoxin-free BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to two weeks or stored at -20°C or colder for up to 3 months.
- Activity
- Cell-Vive™ GMP Recombinant Human TGF-β1 (carrier-free) inhibits the proliferation of mouse HT-2 cells induced by recombinant mouse IL-4 (Cat. No. 574302). The ED50 for this effect is 0.05 – 0.25 ng/mL. The specific activity of Cell-Vive™ GMP Recombinant Human TGF-β1 (carrier-free) is ≥ 1.5 x 107 IU/mg when compared against the WHO International Standard for human TGF-β1 (NIBSC code: 89/514).
- Application
-
Bioassay
- Application Notes
-
Our lyophilized proteins are validated in-house to maintain activity after shipping at ambient temperature and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
- Disclaimer
-
BioLegend Cell-Vive™ GMP Recombinant proteins are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Antigen Details
- Structure
- Dimer
- Function
- TGF-β1 is a multifunctional cytokine that plays pivotal roles in diverse biological processes, including the regulation of cell growth and survival, cell and tissue differentiation, development, inflammation, immunity, hematopoiesis, and tissue remodeling and repair. TGF-β1 is essential for wound healing, stimulates matrix molecule deposition and angiogenesis, and is an essential mediator of the pathologic scarring in fibrotic disorders.
- Interaction
- TGF-beta binding protein (LTBP), epithelial cells, fibroblasts, T cells, B cells, macrophages, and multiple cells respond to TGF-β1.
- Ligand/Receptor
- TGF-β1 binds to type II and type I serine/threonine kinase receptors, which initiate intracellular signals through activation of Smad proteins.
- Bioactivity
- GMP recombinant human TGF-β1 inhibits the proliferation of mouse HT-2 cells induced by recombinant mouse IL-4.
- Cell Sources
- TGF-β1 is secreted by numerous cells. Embryonic Stem Cells, Endothelial cells, Fibroblasts, Mesenchymal cells, Mesenchymal Stem Cells, Neural Stem Cells, Osteoclasts, Th17, Tregs
- Antigen References
-
- Sun X, et al. 2013. J Virol. 87:10126.
- Benlhabib H, et al. 2015. J Biol Chem. 290:22409.
- Lin C, et al. 2016. J Exp Med. 213:251.
- Chen P, et al. 2016. Sci Rep. 6:33407.
- de la Mare JA, et al. 2017. BMC Cancer. 17:202.
- Du C, et al. 2017. Int J Biochem Cell Biol. 90:17.
- Nüchel J, et al. 2018. Autophagy. 14:465.
- Gene ID
- 7040 View all products for this Gene ID
- UniProt
- View information about TGF-beta1 on UniProt.org
 Login / Register
Login / Register 













Follow Us