- Regulatory Status
- RUO
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

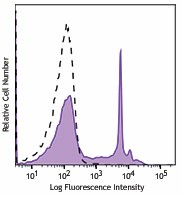
One-day-old C57BL/6 mouse splenocytes were stained with Helix NP™ Blue (filled histogram) at 5nM. Cells alone, without Helix NP™ Blue staining, are also shown (open histogram). -
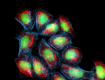
HeLa cells were fixed with 1% paraformaldehyde (PFA) for 10 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes, and blocked with 5% FBS for 30 minutes. Then the cells were intracellularly stained with 5 µg/mL of Alexa Fluor® 647 anti-Cytokeratin (pan reactive) (clone C-11) antibody (red) in blocking buffer overnight followed by 25 µM of Helix NP™ Blue (green) and Flash Phalloidin™ Red 594 (blue) staining for 15 minutes at room temperature. The image was captured with a 60X objective using the Alexa Fluor® 488 filter. -
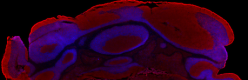
Mouse frozen cerebellum tissue was fixed with 4% paraformaldehyde (PFA) for 10 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes, and blocked with 5% FBS for one hour. Then the tissue was intracellularly stained with 5 µM of Helix NP™ Blue (blue) 15 minutes at room temperature and co-stained with Flash Phalloidin™ Red (red). The image was captured with a 10X objective using the Alexa Fluor® 488 filter.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 425305 | 250 µL | 211 CHF | ||||
Helix NP™ Blue is a blue-emitting nucleic acid stain. It is impermeant to live cells and thus can be used for the discrimination of live and dead cells. In fixed and permeabilized cells, it can be used to assess cell cycle status. In immunofluorescence microscopy, it can be used as a nuclear counterstain in cells and tissue. It is optimally excited at 430 nm with an emission at 470 nm, which can be detected in the Brilliant Violet 421™ or Pacific Blue™ channel.
Product DetailsProduct Details
- Preparation
- Helix NP™ Blue is supplied at 250 µl per vial.
- Concentration
- 5.0 mM
- Storage & Handling
- Upon receipt, store at -20°C.
- Application
-
FC - Quality tested
ICC, IHC-F - Verified - Recommended Usage
-
For flow cytometric viability staining, the suggested use of this reagent is 5 - 200 nM. For flow cytometric fixed cell cycle analysis, the suggested use of this reagent is 0.2 - 20 nM. For immunofluorescence microscopy and immunohistochemical staining on frozen tissue sections, the suggested use of this reagent is 2.5 - 50 µM. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Helix NP™ Blue is a cell-impermeant nucleic acid probe suitable for use as a viability dye and for assessing DNA content and cell cycle status following cell fixation and permeabilization in flow cytometry. It can also be used as a viability dye in live cell imaging or as a nuclear counterstain on fixed and permeabilized cells and tissues. It is a blue-emitting dye with an excitation/emission max of 430 nm/470 nm that can be detected in the Brilliant Violet 421™ or Pacific Blue™ channel.
Protocol for flow cytometric viability staining using Helix NP™ Blue:-
Isolate cells following protocol of choice.
-
Optional— For multicolor flow cytometry experiments, surface-stain cells as recommended. Helix NP Blue should be added as the last step prior to sample acquisition.
-
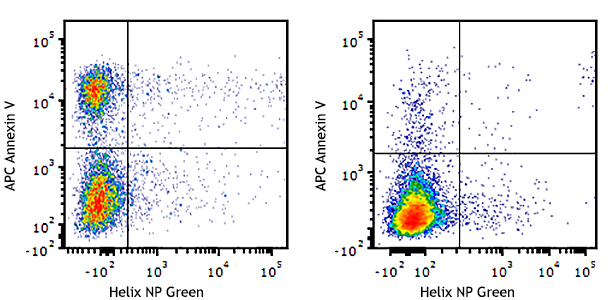
Note: The use of Helix NP for viability staining is incompatible with cell fixation and permeabilization protocols. If cells are to be fixed and permeabilized, use a fixable viability dye, such as a Zombie™ Fixable Viability kit.
-
-
Dilute Helix NP to required concentration. We have observed good flow cytometric viability staining in a final concentration of 5 - 200 nM Helix NP Blue, but recommend titrating the reagent to determine the optimal concentration for cells of interest.
-
For example, to stain cells in a final concentration of 20 nM Helix NP Blue, prepare a 1:5000 dilution of the 5.0 mM stock in Cell Staining Buffer (or equivalent). Then, add 10 µL of diluted reagent to 500 µL of cell suspension.
-
-
Do not wash the cells after adding Helix NP™ Blue. Samples are ready for acquisition.
-
Analyze cells on a cytometer equipped with a 405 nm violet laser.
Protocol for fixed cell cycle analysis using Helix NP™ Blue:
- Isolate cells following protocol of choice.
- Wash cells twice with phosphate-buffered saline (PBS). Using 70% ethanol (EtOH) chilled to -20°C, slowly add to cells while vortexing. Following the addition of 70% EtOH, continue vortexing for an additional 30 seconds. Incubate fixed cells at -20°C for a minimum of 1 hour. Prior to staining, wash cells once with phosphate-buffered saline (PBS), followed by another wash with Cell Staining Buffer (Cat. No. 420201).
- Dilute Helix NP™ Blue to required concentration. We have observed good cell cycle analysis results using a final Helix NP Blue concentration of 0.2 - 20 nM, but recommend titrating the reagent to determine the optimal concentration for cells of interest.
- For example, to stain cells in a final concentration of 20 nM Helix NP Blue, prepare a 1:5000 dilution of the 5.0 mM stock in Cell Staining Buffer (or equivalent). Then, add 10 µL of diluted reagent to 500 µL of cell suspension.
- Do not wash cells after adding Helix NP™ Blue. Samples are ready for acquisition.
- Analyze cells on a cytometer equipped with a 405 nm laser.
Protocol for nuclear counterstaining fixed and permeabilized cell/tissue specimens using Helix NP™ Blue:
- Fix specimens with 1%-4% Paraformaldehyde (PFA) for 10 minutes at room temperature.
- 1% for cultured cells
- 4% for frozen tissue
- Wash the cells/tissue two times with 1X PBS.
- Permeabilize the cells/tissue with 0.5% Triton X-100 for 10 minutes at room temperature.
- Wash the cells/tissue two times with 1X PBS.
- Block cells with 5% fetal bovine serum for 30 minutes at room temperature.
- Complete any additional blocking steps, antibody staining and washes prior to the addition of the Helix NP™ Blue counterstain.
- Prepare the working solution.
- It is best to try a range of dye concentrations to determine the optimal concentration for cells/tissues of interest to achieve the best image. We have obtained good results using a starting range of 2.5 - 50 µM for IHC-F and 2.5 - 25 µM for IF/ICC.
- Stain the cells/tissues with working solution for 20 minutes at 4°C or room temperature in dark.
- Protect from light prior to imaging.
- No wash step is needed after staining.
- Mount the slides with an antifade medium.
- Image slides with a filter ideal for ex/em max of 430 nm/470 nm (either Alexa 488 or BV510 filter)
-
Antigen Details
- Biology Area
- Apoptosis/Tumor Suppressors/Cell Death, Cell Biology, Cell Cycle/DNA Replication, Cell Proliferation and Viability
- Gene ID
- NA
 Login / Register
Login / Register 













Follow Us