- Clone
- MEC13.3 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- PECAM-1, EndoCAM
- Isotype
- Rat IgG2a, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

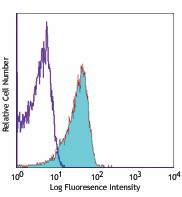
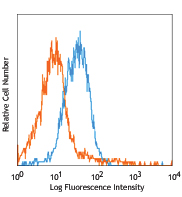
C57BL/6 mouse splenocytes stained with MEC13.3 Alexa Fluor® 647 -

Formalin-fixed, 300 micron-thick mouse kidney section was blocked, permeabilized and stained overnight with CD31 (clone MEC13.3) Alexa Fluor® 647 (red) at 5 µg/mL, Tubulin Beta 3 (TUBB3)(clone AA10) Alexa Fluor® 488 (green) at 2.5 µg/mL, and Podoplanin (clone Pmab-1) Alexa Fluor® 594 (blue) at 5 µg/mL, optically cleared, then analyzed at 230 µm imaging depth on a confocal microscope. Watch the video. -
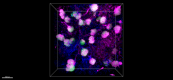
Paraformaldehyde-fixed (4%), 500 μm-thick mouse kidney section was processed according to the Ce3DTM Tissue Clearing Kit protocol (cat. no. 427701). The section was costained with anti-mouse Podoplanin Antibody (clone PMab-1) Alexa Fluor® 594 at 5 µg/mL (green), and anti-mouse CD31 Antibody (clone MEC13.3) Alexa Fluor® 647 at 5 µg/mL (magenta) and counterstained with DAPI (blue). The section was then optically cleared and mounted in a sample chamber. The image was captured with a 20X objective using Zeiss 780 confocal microscope and processed by Imaris image analysis software. -
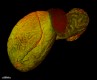
In-vivo mouse testis labeling of blood vessels with Alexa Fluor® 647 anti-mouse CD31 (red, clone MEC13.3). Animal perfused and sample dehydrated and cleared with ECi. Autofluorescence (green). Image generously submitted to the 2017 Cell Life Imaging Competition by Simon Merz from University of Duisburg-Essen. -
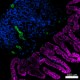
Confocal image of C57BL/6 mouse small intestine sample acquired using the IBEX method of highly multiplexed antibody-based imaging: EpCAM (magenta) in Cycle 1, CD8 (blue) in Cycle 1, and CD31 (green) in Cycle 2. Tissues were prepared using ~1% (vol/vol) formaldehyde and a detergent. Following fixation, samples are immersed in 30% (wt/vol) sucrose for cryoprotection. Images are courtesy of Drs. Andrea J. Radtke and Ronald N. Germain of the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH). -
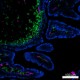
Confocal image of C57BL/6 mouse small intestine sample acquired using the IBEX method of highly multiplexed antibody-based imaging: EpCAM (blue) in Cycle 1, CD31 (magenta) in Cycle 2, and MHCII (green) in Cycle 3. Tissues were prepared using ~1% (vol/vol) formaldehyde and a detergent. Following fixation, samples are immersed in 30% (wt/vol) sucrose for cryoprotection. Images are courtesy of Drs. Andrea J. Radtke and Ronald N. Germain of the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH).
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 102515 | 25 µg | 81€ | ||||
| 102516 | 100 µg | 184€ | ||||
CD31 is a 130-140 kD glycoprotein, also known as platelet endothelial cell adhesion molecule (PECAM-1), EndoCAM, and gpIIa. It is a member of the Ig superfamily, expressed on endothelial cells, platelets, granulocytes, monocytes/macrophages, dendritic cells, and T and B cell subsets, and is critical for cell-to-cell interactions. The primary ligands for CD31 have been reported to be CD38 and the vitronectin receptor (αv β3 integrin, CD51/CD61). Other reported functions of CD31 are neutrophil emigration to sites of inflammation, and angiogenesis.
Product DetailsProduct Details
- Verified Reactivity
- Mouse
- Antibody Type
- Monoclonal
- Host Species
- Rat
- Immunogen
- Polyoma middle T transformed EC line tEnd.1
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide.
- Preparation
- The antibody was purified by affinity chromatography and conjugated with Alexa Fluor® 647 under optimal conditions.
- Concentration
- 0.5 mg/mL
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C, and protected from prolonged exposure to light. Do not freeze.
- Application
-
FC - Quality tested
3D IHC - VerifiedSB - Reported in the literature, not verified in house
- Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 0.25 µg per 106 cells in 100 µL volume. For 3D immunohistochemistry on formalin-fixed tissues, a concentration of 5.0 µg/mL is suggested. It is recommended that the reagent be titrated for optimal performance for each application.
* Alexa Fluor® 647 has a maximum emission of 668 nm when it is excited at 633nm / 635nm.
Alexa Fluor® and Pacific Blue™ are trademarks of Life Technologies Corporation.
View full statement regarding label licenses - Excitation Laser
-
Red Laser (633 nm)
- Application Notes
-
Anti-mouse CD31 clones 390 and MEC13.3 bind to their respective non-overlapping epitopes in IgD2 of CD31.8 Additional reported applications (in the relevant formats) include: immunoprecipitation1, in vitro and in vivo blocking of CD31-mediated cell-cell interactions1-4, immunohistochemical staining1,5,6 of acetone-fixed frozen sections and zinc-fixed paraffin-embedded sections, and spatial biology (IBEX)12,13.
Special Note: The antibody works well on acetone-fixed frozen sections as well as Zinc-fixed paraffin-embedded sections. It sometime works on formalin-fixed and paraformaldehyde-fixed paraffin-embedded tissue sections but inconsistent results have been reported. This antibody is not recommended for formalin-fixed paraffin-embedded sections or for Western blot analysis. The Ultra-LEAF™ Purified antibody (Endotoxin < 0.01 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for functional assays (Cat. No. 102529 and 102530). -
Application References
(PubMed link indicates BioLegend citation) -
- Vecchi A, et al. 1994. Eur. J. Cell Biol. 63:247. (IP, IHC, Block)
- Christofidou-Solomidou M, et al. 1997. J. Immunol. 158:4872. (Block)
- DeLisser HM, et al. 1997. Am. J. Pathol. 151:671. (Block)
- Rosenblum WI, et al. 1994. Am. J. Pathol. 145:33. (Block)
- Baldwin HS, et al. 1994. Development 120:2539. (IHC)
- Voswinckel R, et al. 2003. Circ. Res. 93:372. (IHC)
- Leung VW, et al. 2009. Am J. Pathol. 175:1757. PubMed
- Chacko AM, et al. 2012. PLoS One 7:e34958.
- Giacomini C, et al. 2014. Exp Eye Res. 18:1. PubMed
- Morita R, et al. 2015. PNAS. 112:160. PubMed
- Ito A, et al. 2015. Brain Res. 1594:310. PubMed
- Radtke AJ, et al. 2020. Proc Natl Acad Sci U S A. 117:33455-65. (SB) PubMed
- Radtke AJ, et al. 2022. Nat Protoc. 17:378-401. (SB) PubMed
- Product Citations
-
- RRID
-
AB_2161030 (BioLegend Cat. No. 102515)
AB_2161029 (BioLegend Cat. No. 102516)
Antigen Details
- Structure
- Ig superfamily, 130-140 kD
- Distribution
-
Endothelial cells, platelets, granulocytes, monocytes/macrophages, dendritic cells, T and B cell subsets
- Function
- Adhesion
- Ligand/Receptor
- CD38, αV/β3 integrin
- Cell Type
- B cells, Dendritic cells, Endothelial cells, Granulocytes, Macrophages, Monocytes, Neutrophils, Platelets, T cells
- Biology Area
- Angiogenesis, Cell Adhesion, Cell Biology, Immunology, Neuroinflammation, Neuroscience
- Molecular Family
- Adhesion Molecules, CD Molecules
- Antigen References
-
1. Barclay AN, et al. 1997. The Leukocyte Antigen FactsBook Academic Press.
2. DeLisser HM, et al. 1994. Immunol. Today 15:490.
3. Newman PJ, et al. 1990. Science 247:1219. - Gene ID
- 18613 View all products for this Gene ID
- Specificity (DOES NOT SHOW ON TDS):
- CD31
- Specificity Alt (DOES NOT SHOW ON TDS):
- CD31
- App Abbreviation (DOES NOT SHOW ON TDS):
- FC,3D IHC,SB
- Hidden Names (DOES NOT SHOW ON TDS):
- PECAM1, PECAM 1, Endothelial Cell, Megakarocyte, Neutrophil, Platelets, Stromal Cells
- UniProt
- View information about CD31 on UniProt.org
Related FAQs
- If an antibody clone has been previously successfully used in IBEX in one fluorescent format, will other antibody formats work as well?
-
It’s likely that other fluorophore conjugates to the same antibody clone will also be compatible with IBEX using the same sample fixation procedure. Ultimately a directly conjugated antibody’s utility in fluorescent imaging and IBEX may be specific to the sample and microscope being used in the experiment. Some antibody clone conjugates may perform better than others due to performance differences in non-specific binding, fluorophore brightness, and other biochemical properties unique to that conjugate.
- Will antibodies my lab is already using for fluorescent or chromogenic IHC work in IBEX?
-
Fundamentally, IBEX as a technique that works much in the same way as single antibody panels or single marker IF/IHC. If you’re already successfully using an antibody clone on a sample of interest, it is likely that clone will have utility in IBEX. It is expected some optimization and testing of different antibody fluorophore conjugates will be required to find a suitable format; however, legacy microscopy techniques like chromogenic IHC on fixed or frozen tissue is an excellent place to start looking for useful antibodies.
- Are other fluorophores compatible with IBEX?
-
Over 18 fluorescent formats have been screened for use in IBEX, however, it is likely that other fluorophores are able to be rapidly bleached in IBEX. If a fluorophore format is already suitable for your imaging platform it can be tested for compatibility in IBEX.
- The same antibody works in one tissue type but not another. What is happening?
-
Differences in tissue properties may impact both the ability of an antibody to bind its target specifically and impact the ability of a specific fluorophore conjugate to overcome the background fluorescent signal in a given tissue. Secondary stains, as well as testing multiple fluorescent conjugates of the same clone, may help to troubleshoot challenging targets or tissues. Using a reference control tissue may also give confidence in the specificity of your staining.
- How can I be sure the staining I’m seeing in my tissue is real?
-
In general, best practices for validating an antibody in traditional chromogenic or fluorescent IHC are applicable to IBEX. Please reference the Nature Methods review on antibody based multiplexed imaging for resources on validating antibodies for IBEX.
Other Formats
View All CD31 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| APC anti-mouse CD31 | MEC13.3 | FC |
| Biotin anti-mouse CD31 | MEC13.3 | FC |
| FITC anti-mouse CD31 | MEC13.3 | FC |
| PE anti-mouse CD31 | MEC13.3 | FC |
| Purified anti-mouse CD31 | MEC13.3 | FC,IP,Block,IHC-F |
| Alexa Fluor® 488 anti-mouse CD31 | MEC13.3 | FC |
| Alexa Fluor® 647 anti-mouse CD31 | MEC13.3 | FC,3D IHC,SB |
| Alexa Fluor® 594 anti-mouse CD31 | MEC13.3 | IHC-F,FC,3D IHC,SB |
| PerCP/Cyanine5.5 anti-mouse CD31 | MEC13.3 | FC |
| PE/Cyanine7 anti-mouse CD31 | MEC13.3 | FC |
| PE/Dazzle™ 594 anti-mouse CD31 | MEC13.3 | FC |
| APC/Fire™ 750 anti-mouse CD31 | MEC13.3 | FC |
| Ultra-LEAF™ Purified anti-mouse CD31 | MEC13.3 | FC,IP,Block,IHC-F |
| APC/Cyanine7 anti-mouse CD31 | MEC13.3 | FC |
| Spark YG™ 570 anti-mouse CD31 | MEC13.3 | IHC-F |
| Spark Red™ 718 anti-mouse CD31 (Flexi-Fluor™) | MEC13.3 | FC |
Customers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
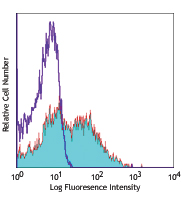
APC anti-mouse CD31
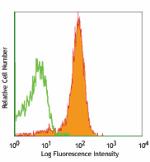
C57BL/6 splenocytes stained with MEC13.3 APC -
Biotin anti-mouse CD31
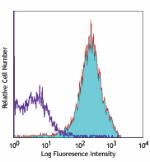
C57BL/6 mouse splenocytes stained with biotinylated MEC13.3,... -
FITC anti-mouse CD31
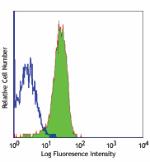
C57BL/6 mouse splenocytes stained with MEC13.3 FITC -
PE anti-mouse CD31
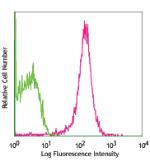
C57BL/6 mouse splenocytes stained with MEC13. 3 PE -
Purified anti-mouse CD31
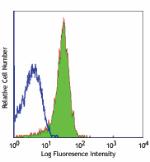
C57BL/6 mouse splenocytes stained with purified MEC13.3, fol... -
Alexa Fluor® 488 anti-mouse CD31
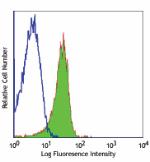
C57BL/6 mouse splenocytes stained with MEC13.3 Alexa Fluor® ... -
Alexa Fluor® 647 anti-mouse CD31
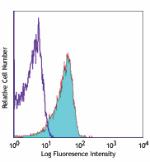
C57BL/6 mouse splenocytes stained with MEC13.3 Alexa Fluor® ... 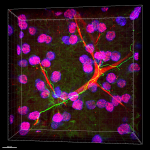
Formalin-fixed, 300 micron-thick mouse kidney section was bl... 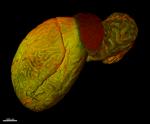
In-vivo mouse testis labeling of blood vessels with Alexa Fl... 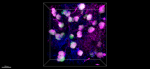
Paraformaldehyde-fixed (4%), 500 μm-thick mouse kidney secti... 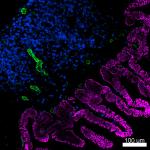
Confocal image of C57BL/6 mouse small intestine sample acqui... 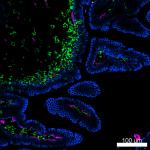
Confocal image of C57BL/6 mouse small intestine sample acqui... -
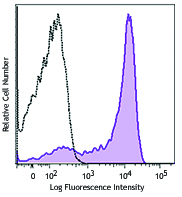
Alexa Fluor® 594 anti-mouse CD31
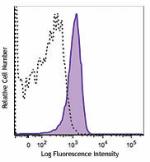
C57BL/6 mouse splenocytes were stained with CD31 (clone MEC1... 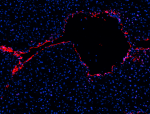
C57BL/6 mouse frozen liver section was fixed with 4% parafor... 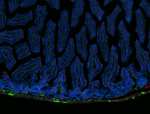
Dissected C57/B6 mouse stomach was immersed in 4% paraforma... 
Paraformaldehyde-fixed (4%), 500 μm-thick mouse kidney secti... 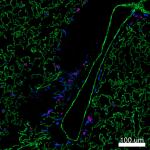
Confocal image of C57BL/6 mouse lung sample acquired using t... 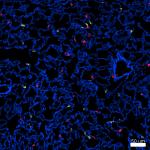
Confocal image of C57BL/6 mouse lung sample acquired using t... -
PerCP/Cyanine5.5 anti-mouse CD31
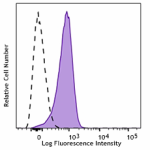
C57BL/6 mouse splenocytes were stained with CD31 (clone MEC1... -
PE/Cyanine7 anti-mouse CD31
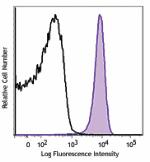
C57BL/6 mouse splenocytes were stained with CD31 (clone MEC1... -
PE/Dazzle™ 594 anti-mouse CD31
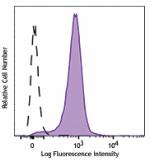
MEC13dot3_PEDZZL594_CD31_Antibody_040517 -
APC/Fire™ 750 anti-mouse CD31
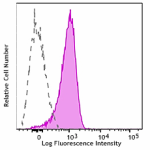
C57BL/6 were stained with CD31 (clone MEC13.3) APC/Fire™ 750... -
Ultra-LEAF™ Purified anti-mouse CD31
-
APC/Cyanine7 anti-mouse CD31
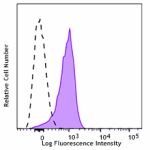
C57BL/6 mouse splenocytes were stained with CD31 (clone MEC1... -
Spark YG™ 570 anti-mouse CD31
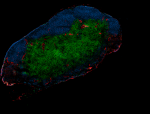
C57BL/6 mouse frozen lymph node section was fixed with 4% pa... -
Spark Red™ 718 anti-mouse CD31 (Flexi-Fluor™)
 Login / Register
Login / Register 














Follow Us