- Clone
- 6E10 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- AAA, ABETA, ABPP, AD1, APPI, CTFgamma, CVAP, PN-II, PN2, Amyloid beta A4 protein, preA4, protease, peptidase nexin-II, beta-amyloid peptide, alzheimer disease amyloid protein, cerebral vascular amyloid peptide, APP, Amyloid Precursor Protein
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
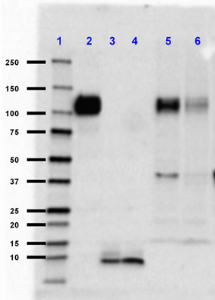
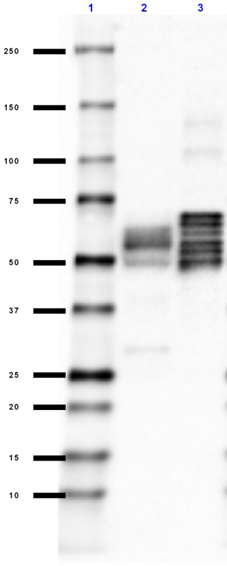
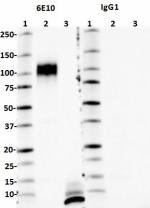
Western blot of Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 antibody (clone 6E10). Lane 1: Molecular weight marker; Lane 2: 10 ng of the recombinant human APP770 protein; Lane 3: 50 ng of the human Aβ1-40 peptide; Lane 4: 50 ng of the human Aβ1-42 peptide; Lane 5: 50 µg of normal human brain lysate; Land 6: 50 µg of Alzheimer's disease human brain lysate. The blot was incubated with 1 µg/mL of the primary antibody overnight at 4°C, followed by incubation with HRP labeled goat anti-mouse IgG (Cat. No. 405306). Enhanced chemiluminescence was used as the detection system. -
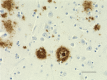
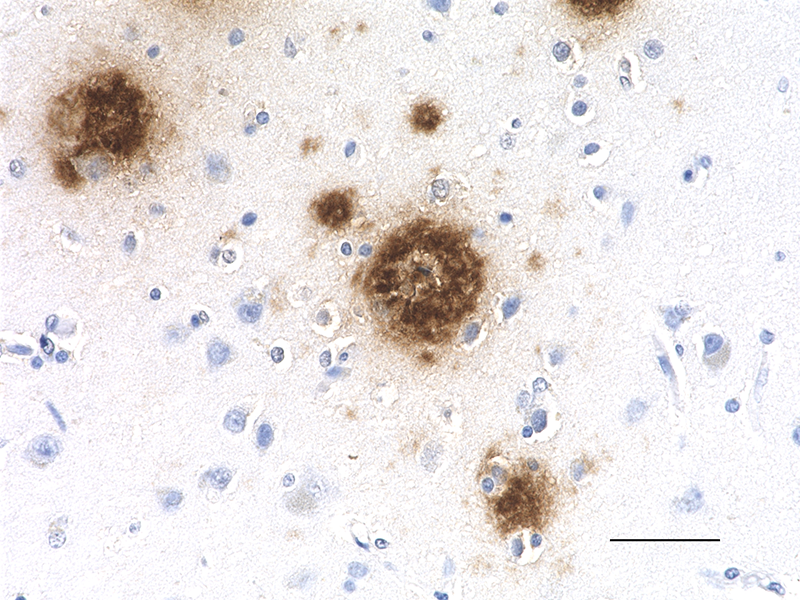
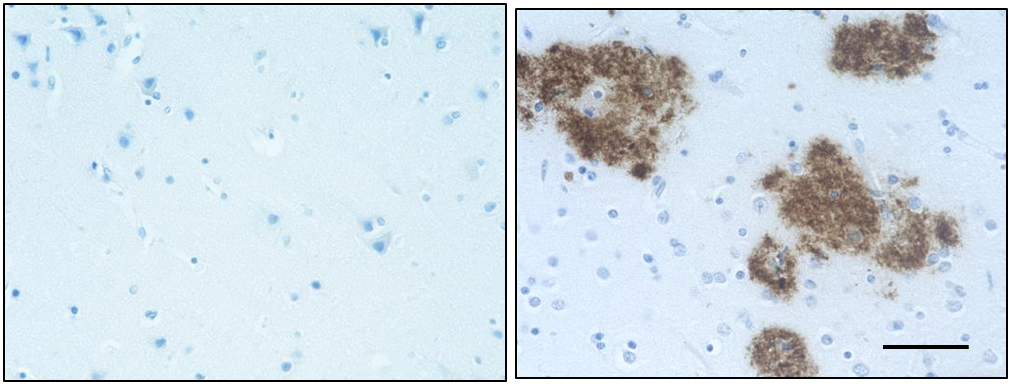
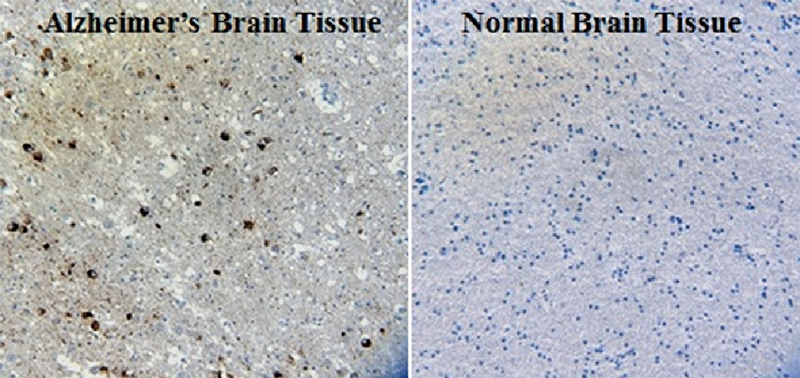
IHC staining of Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 antibody (clone 6E10) on formalin-fixed paraffin-embedded human Alzheimer's disease brain tissue. Following antigen retrieval using 88% formic acid for 20 minutes at room temperature, the tissue was incubated with 1 µg/ml of the primary antibody for 60 minutes at room temperature. BioLegend´s Ultra-Streptavidin (USA) HRP kit (Multi-Species, DAB, Cat. No. 929901) was used for detection followed by hematoxylin counterstaining, according to the protocol provided. The image was captured with a 40X objective. Scale bar: 50 µm -
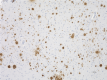
IHC staining of Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 antibody (clone 6E10) on formalin-fixed paraffin-embedded human Alzheimer's disease brain tissue. Following antigen retrieval using 88% formic acid for 20 minutes at room temperature, the tissue was incubated with 1 µg/ml of the primary antibody for 60 minutes at room temperature. BioLegend´s Ultra-Streptavidin (USA) HRP kit (Multi-Species, DAB, Cat. No. 929901) was used for detection followed by hematoxylin counterstaining, according to the protocol provided. The image was captured with a 10X objective. Scale bar: 50 µm
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 803024 | 1 mg | 1605€ | ||||
| 803023 | 100 µg | 269€ | ||||
Alzheimer's disease is characterized by the accumulation of aggregated Aβ peptides in senile plaques and vascular deposits. Aβ peptides are derived from amyloid precursor proteins (APP) through sequential proteolytic cleavage of APP by β-secretases and γ-secretases generating diverse Aβ species. Aβ can aggregate to form soluble oligomeric species and insoluble fibrillar or amorphous assemblies. Some forms of the aggregated peptides are toxic to neurons.
Product DetailsProduct Details
- Verified Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- Less than 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
WB - Quality tested
IHC-P - Verified
ICC, IHC-F, EM - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by Western blotting. For Western blotting, the suggested use of this reagent is 0.2 - 1.0 µg per mL. For immunohistochemistry on formalin-fixed paraffin-embedded tissue, a concentration range of 0.2 - 5.0 µg/mL is suggested. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
This antibody is reactive to amino acid residue 1-16 of beta amyloid. The epitope lies within amino acids 3-8 of beta amyloid (EFRHDS).
This antibody clone has been reported for use in immunohistochemistry of free-floating sections2,13. -
Application References
(PubMed link indicates BioLegend citation) -
- Thakker DR, et al. 2009. Proc. Natl. Acad. Sci. USA. 106(11):4501-6. (IHC) PubMed
- Oddo S, et al. 2005. Proc. Natl. Acad. Sci. USA. 102(8):3046-51. (IHC-other) PubMed
- Herzig M, et al. 2004. Nat. Neuro. 7(9):954-959. (WB) PubMed
- Zheng Y, et al. 2012. PLoS One 6:39035. (IHC-F) PubMed
- Abramowksi D, et al. J Neurosci. 32:1273. (WB) PubMed
- Forny-Germano L, et al. 2014. J. Neurosci. 34:13629. (WB, IHC) PubMed
- Gowert NS, et al. 2014. PLoS One 2:e90523. (ICC, EM) PubMed
- Sandoval-Hernandez A, et al. 2015. PLoS One. 10: 0145467. (IHC-F)
- Kumar R, et al. 2016. Brain. 139:174-92 (WB)
- Miyamoto T, et al. 2016. J. Biol. Chem. 291:1719-34. (WB)
- Saito S, et al. 2017. Acta Neuropathol. Commun. 5:26-9. (IHC-P) PubMed
- Omata Y, et al. 2016. Aging (Albany NY) 8(3):427. (IHC-P) PubMed
- Peng W, et al. 2016. Neurobiol. Dis. 93:215. (IHC-other) PubMed
- Mandler M, et al. 2015. PLoS One. e0115237. (WB, IHC, ELISA) PubMed
- Product Citations
-
- RRID
-
AB_2810701 (BioLegend Cat. No. 803024)
AB_2810700 (BioLegend Cat. No. 803023)
Antigen Details
- Structure
- Amyloid precursor protein is a 770 amino acid protein with a molecular mass of ~100 kD. According to the UniProtKB database, APP (ID# P05067) has 11 isoforms (34 to ~90 kD) and the 770 form has been designated as the canonical form. Isoform APP695 is the predominant form expressed in neuronal tissue. Isoforms APP751 and APP770 are widely expressed in non-neuronal cells. Isoform APP751 is the most abundant form in T-lymphocytes. Aβ denotes peptides of 36-43 amino acids generated from cleavage of APP by secr
- Distribution
-
Tissue distribution: Primarily nervous system, but also adipose tissue, intestine, muscle.
Cellular distribution: Cytosol, endosomes, nucleus, plasma membrane, extracellular, and golgi apparatus. - Function
- The normal function of Aβ is not well understood. Several potential physiological roles have been proposed, including: activation of kinase enzymes; protection against oxidative stress; regulation of cholesterol transport; transcription factor, and as an anti-microbial agent.
- Biology Area
- Cell Biology, Neurodegeneration, Neuroscience, Protein Misfolding and Aggregation
- Molecular Family
- APP/β-Amyloid
- Antigen References
-
- Kumar A, et al. 2015. Pharmacol. Rep. 67(2):195.
- Sadigh-Eteghad S, et al. 2015. Med. Princ. Pract. 24(1):1
- Hampel H, et al. 2015. Expert Rev. Neurother. 15(1):83.
- Puig KL, et al. 2012. Exp. Gerontol. 48(7): 608.
- Selkoe DJ, et al. 2016. EMBO Mol. Med. 8(6):595.
- Walsh DM, et al. 2007. J. Neurochem. 101(5):1172.
- Gene ID
- 351 View all products for this Gene ID
- UniProt
- View information about beta-Amyloid 1-16 on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Other Formats
View All β-Amyloid, 1-16 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Alexa Fluor® 488 anti-β-Amyloid, 1-16 | 6E10 | IHC-P |
| Anti-β-Amyloid, 1-16 | 6E10 | WB,Direct ELISA,IHC-P,IHC-F,EM |
| Biotin anti-β-Amyloid, 1-16 | 6E10 | IHC-P |
| Purified anti-β-Amyloid, 1-16 | 6E10 | WB,Direct ELISA,IHC-P,IHC-F,EM,ICC |
| HRP anti-β-Amyloid, 1-16 | 6E10 | IHC-P,WB |
| Alexa Fluor® 594 anti-β-Amyloid, 1-16 | 6E10 | IHC-P |
| Alexa Fluor® 647 anti-β-Amyloid, 1-16 | 6E10 | IHC-P |
| Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 | 6E10 | WB,IHC-P,ICC,IHC-F,EM |
| Spark YG™ 570 anti-β-Amyloid, 1-16 | 6E10 | IHC-P |
Customers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Alexa Fluor® 488 anti-β-Amyloid, 1-16
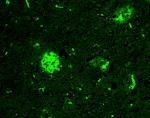
IHC staining of Alexa Fluor® 488 anti-β amyloid, 1-16 antibo... -
Anti-β-Amyloid, 1-16
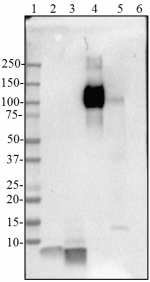
Western blot of anti-β-amyloid, 1-16 antibody (clone 6E10). ... 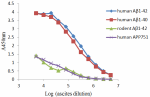
Direct ELISA of anti-β-amyloid, 1-16 (clone 6E10) antibody b... 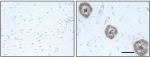
IHC staining of anti-β-Amyloid, 1-16 antibody (clone 6E10) o... -
Biotin anti-β-Amyloid, 1-16
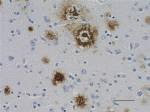
IHC staining of biotin anti-β-Amyloid, 1-16 antibody (clone ... -
Purified anti-β-Amyloid, 1-16
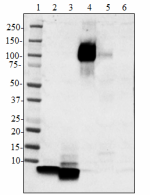
Western blot of purified anti-β-amyloid, 1-16 antibody (clon... 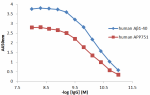
Direct ELISA of purified anti-β-amyloid 1-16 (clone 6E10) an... 
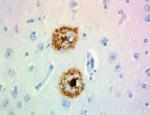
IHC staining of purified anti-β-Amyloid, 1-16 antibody (clo... -
HRP anti-β-Amyloid, 1-16
IHC staining of HRP anti-β-Amyloid, 1-16 antibody (clone 6E1... Western blot of HRP anti-β-Amyloid, 1-16 antibody (clone 6E1... -
Alexa Fluor® 594 anti-β-Amyloid, 1-16
IHC staining of Alexa Fluor® 594 anti-β amyloid, 1-16 antibo... -
Alexa Fluor® 647 anti-β-Amyloid, 1-16
IHC staining of Alexa Fluor® 647 anti-β-Amyloid, 1-16 antibo... 
IHC staining of Alexa Fluor® 647 anti-β-Amyloid, 1-16 antibo... -
Ultra-LEAF™ Purified anti-β-Amyloid, 1-16
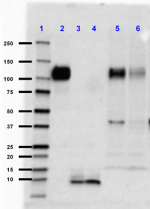
Western blot of Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 an... 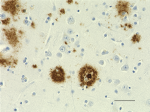
IHC staining of Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 an... 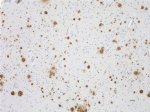
IHC staining of Ultra-LEAF™ Purified anti-β-Amyloid, 1-16 an... -
Spark YG™ 570 anti-β-Amyloid, 1-16
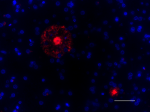
IHC staining of Spark YG™ 570 anti-β-Amyloid, 1-16 antibody ...
 Login / Register
Login / Register 








Follow Us