A Guide to Tandem Dyes and Degradation
| In this blog, we'll describe the common characteristics of tandem dyes, discuss their advantages in multicolor flow cytometry, and address a common myth about tandem degradation. What is a tandem dye? A tandem dye is composed of two covalently attached fluorescent molecules. One of these molecules is either a protein-based (i.e., PE, APC) or synthetic dye (i.e., BV421™) characterized by a large extinction coefficient (capacity to absorb energy) that serves as a donor, while the second molecule is a small synthetic dye that serves as an acceptor (i.e., Cyanine5, Cyanine7). Why do we use tandem dyes? As you may already know, the number of fluorophores one is able to use at the same time is limited by the number of lasers and detectors on the cytometer (Table 1). The most advanced cytometer instruments on the current market have 5 lasers including ultraviolet (355 nm), violet (405 nm), blue (488 nm), green (532 nm) or yellow-green (561 nm), and red (633 nm) and the potential to integrate 8 parameters off of each laser. However, there are not currently enough spectrally distinct fluorophores yet to fill out this configuration. Without tandem fluorophores, each laser would only be suitable to discretely excite a small number of fluorophores, thus severely limiting the total number of parameters able to be detected. |
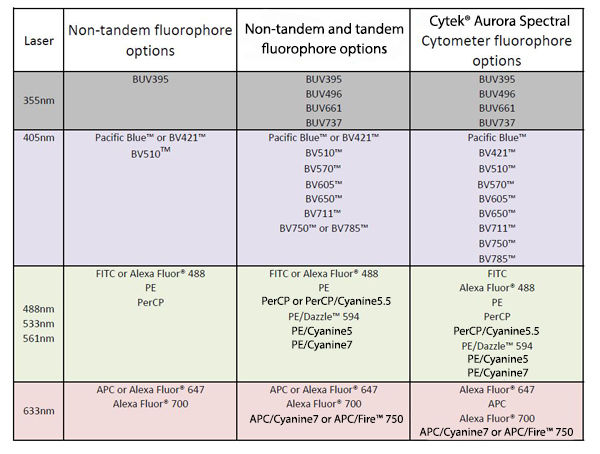 Table 1. Example of multicolor flow panel diversification based on available lasers, detectors, and instrument. |
| Further expansion of the number of detectors off of each laser has allowed us to increase flow panels up to 21 colors /23 parameters (Table 1). It is possible to achieve because the tandem dyes use the same excitation characteristics as the donor dye but possess emission properties of the acceptor. Therefore, though donor dyes and their tandems are excited by the same laser, they can be used in the same panel by utilizing different detectors to readout their emission. As an example, Figure 1 demonstrates the excitation and emission spectrum of BV421™ and its tandems. The further expansion of multicolor panels can be achieved by using a spectral cytometer, such as the Cytek® Aurora Spectral Cytometer. This instrument has 48 fluorescent channels with three lasers. Theoretically, it can detect 42 fluorophores as long as those fluorophores have distinct emission profiles. A spectral detection system does not utilize traditional methods of compensation calculation and allows a simultaneous use of fluorophores with significant overlapping emission spectrum (i.e. BV750™ and BV785™) while having minimal compensation concerns. |
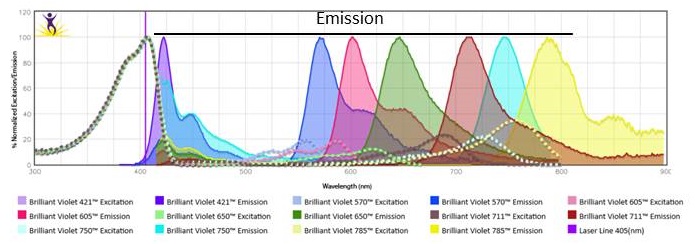 Figure 1. Excitation and emission spectra of BV421™ and its tandems, BV570™, BV605™, BV650™, BV711™, BV750™, BV785™. |
| How do tandem dyes work? The donor fluorophore absorbs light energy of the specific wavelength. Upon excitation, energy is transferred from the donor to the acceptor through a phenomenon called Förster resonance energy transfer (FRET), also known as fluorescence resonance energy transfer. The acceptor emits the transferred energy as fluorescent light. When FRET efficiency is high, a strong signal will be observed in the acceptor channel and a weaker signal in the donor channel. Please note, the FRET efficiency is never 100%, which means that some spillover or bleeding in the donor channel is expected. I see a fluorescence signal in the donor channel. Is my tandem degrading? NO. This is a common myth. The donor and acceptor dyes are covalently conjugated and don't typically fall apart. As mentioned earlier some spillover or bleeding into the donor channel is expected since FRET efficiency is not 100%. To accurately compensate spillover, it is important to use the same antibody used in the multicolor sample as the single color compensation control and to treat them identically as the multicolor sample especially in light and fixative exposure. However, if you observe a strong signal in the donor channel and a weak signal in the acceptor channel, this is an indication of low FRET efficiency that is caused by one of the following factors: |
|
| As an example of how different fixatives may affect the fluorescence signal of tandem dyes, Figure 2 demonstrates the fluorescence signal from cells stained with a PerCP/Cyanine5.5-conjugated antibody and exposed to 1%PFA or True-Phos™ Perm buffer. Exposure to the True-Phos™ Perm buffer, which is methanol-based, leads to the denaturation of PerCP since this is a protein based dye. It results in the loss of signal for PerCP but not for its acceptor, Cyanine5.5 whose fluorescence can be detected in the Alexa Fluor® 700 channel. |
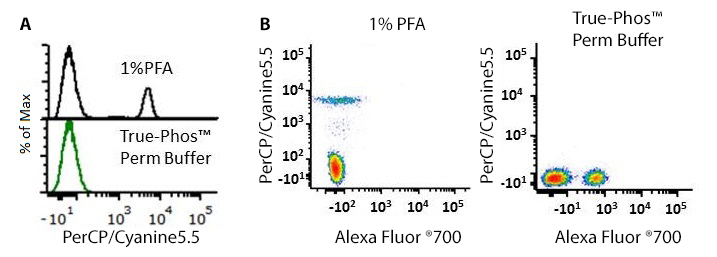 Figure 2. A) Fluorescence intensity of PerCP/Cyanine5.5 anti-CD14 antibody staining upon exposure to 1% PFA or True-Phos™ Perm buffer solution. B) Dot plots demonstrating appearance of signal in Alexa Fluor® 700 channel upon exposure of PerCP/Cyanine5.5 to True-Phos™ Perm buffer. |
| Hopefully, now you have a better understanding of tandem dyes and are familiar with guidelines on their use and handling when planning and doing your experiments. As always, if you have any questions while planning your experiments, feel free to reach out to our technical services team at tech@biolegend.com. You can learn more about these fluorophores with our tandem dyes webpage. |
| Contributed by Ekaterina Zvezdova, PhD. |
| View a full listing of Trademarks and Patents. |
 Login / Register
Login / Register 






Follow Us