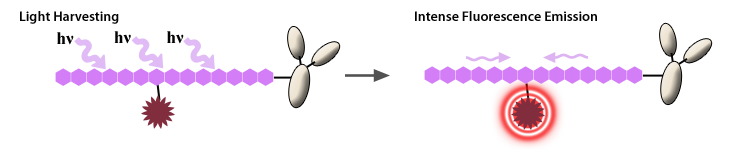
A tandem is composed of two covalently attached fluorescent molecules (one of which serves as the donor and the other as acceptor) that behaves as a unique fluorophore with the excitation properties of the donor and the emission properties of the acceptor. This is possible through the phenomenon of Förster resonance energy transfer (FRET), also known as fluorescence resonance energy transfer. This allows one fluorophore to pass its excitation energy to a neighboring fluorophore, which then emits the photon of light. This transfer of energy is dependent on the proximity and orientation of the donor and acceptor molecules.

Tandem Dyes
Important Notes:
- FRET efficiency is never 100%, which means that there will always be some bleedthrough emission from the donor that is observed. This is dependent on the quality of the tandem conjugates. Be sure to use isotype controls and single color controls to appropriately adjust your compensation settings.
- Tandems are commonly known for ”degradation“ or ”decoupling“, describing the loss of emission from the acceptor and increased emission by the donor. Because tandems are covalently attached, the donor and acceptor do not typically come apart in solution. There are potentially several causes that induce the loss of FRET to the acceptor. The first is exposure to light which is known to photobleach both the donor/acceptor molecules. Oxygen radicals also cause damage to the acceptor dyes. Both conditions would be irreversible and would confer loss of function to the tandem.
- When using tandems in multicolor flow cytometry, it is critical to use isotype controls for background, single color controls for compensation, and FMO (Fluorescence-minus-one) controls for gating.

Secrets to successful tandem use:
- Avoid extended exposure to light and extreme variations in temperature. Light will photobleach your tandem – protect your samples and antibody from light at all times. Do not freeze your tandem antibody conjugates, as this could potentially lead to denaturing of the donor protein.
- Avoid ”overfixing“. We recommend that tandems be fixed for short-periods of time (less than 30 minutes) and then washed and resuspended in Cell Staining buffer for storage. For this purpose, we provide specially formulated FluoroFix™ buffer, for the fixation of tandem dyes.
- Tandem Stabilizer helps to reduce tandem dye de-coupling when added as a supplement to buffers commonly used to store stained, fixed cells, as well as those used for fixation and permeabilization procedures.
- Be aware of cross-beam excitation of your acceptor molecule when performing multi-laser flow cytometry with tandems. In most cases, this can be compensated. For example, the Cyanine5 acceptor in PE/Cyanine5 is known to be excitable by the red laser, so use caution in combination with APC.
- Label your cells at 4°C or on ice. APC tandems may be susceptible to cell-mediated decoupling, so slowing down cell metabolism at low temperatures can help preserve the stability of the tandem.
Historically, tandem dyes were composed of a large protein donor, such as PE or APC, attached to a small molecule acceptor. Now, with the introduction of Brilliant Violet™ polymers, a new family of tandems can be classified. These Brilliant Violet™ tandems do not require the emission of the donor to overlap with the excitation of the acceptor. This allows for tandem molecules based on the Brilliant Violet 421™ donor with extremely high stokes shifts, emitting as far out as 785 nm with a 405 nm excitation. The families are compared below:
| Phycobiliprotein tandems | Brilliant Violet™ tandems |
|---|---|
| Donor: PE, APC, PerCP | Donor: Brilliant Violet 421™ |
| Typically requires overlap of donor emission and acceptor excitation. | No overlap of donor emission and acceptor excitation required. |
| Protein component susceptible to fixation and degradation. | No protein, so less susceptible to denaturation. |
| PE and PerCP tandems are relatively bright. APC tandems tend to be relatively dim. | Brilliant Violet™ tandems are bright, ranging from 3-5 on a scale of 1-5, with 5 being brightest. |
| PE/Cyanine5 and PE/Cyanine7 are known to bind non-specifically to monocytes. With improved performance, PE/Dazzle™ 594 has considerably less nonspecific binding. | No known non-specific binding. |
Lot-to-Lot Consistency in Manufacturing
It is critical that tandems are manufactured consistently and provide dependable results over time. BioLegend takes extra care in manufacturing high quality tandem products, using strict specifications for each tandem that we produce, including donor:acceptor ratio, f/p ratio, and minimal bleedthrough of the donor. We verify lot-to-lot performance with flow cytometry quality control testing pre-bottling and post-bottling against a gold standard. Below are a few representative comparisons of consistent tandem performance from lot-to-lot. It is important to note that variations in tandem performance can vary widely from vendor to vendor and even potentially, from lot-to-lot with the same vendor. For this reason, we recommend using isotype controls from the same vendor whenever possible.
BioLegend available tandems
| Tandem | Exmax(nm) | Emmax(nm) | Bandpass Filter | Brightness |
|---|---|---|---|---|
| Brilliant Violet 570™ | 405 | 570 | 585/42 | 1 |
| Brilliant Violet 605™ | 405 | 603 | 610/20 | 3 |
| Brilliant Violet 650™ | 405 | 646 | 660/20 | 3 |
| Brilliant Violet 711™ | 405 | 711 | 710/50 | 4 |
| Brilliant Violet 750™ | 405 | 750 | 780/60 | 3 |
| Brilliant Violet 785™ | 405 | 785 | 780/60 | 3 |
| PerCP/Cyanine5.5 | 482 | 695 | 710/50 | 2 |
| PerCP/Fire™ 780 | 488 | 774 | 780/60 | 4 |
| PerCP/Fire™ 806 | 488 | 806 | 780/60 | 4 |
| PE/Dazzle™ 594 | 565 | 610 | 610/20 | 5 |
| PE/Fire™ 640 | 565 | 639 | 660/20 | 4 |
| PE/Cyanine5 | 565 | 667 | 660/20 | 5 |
| PE/Fire™ 700 | 565 | 695 | 670/30 | 5 |
| PE/Fire™ 744 | 565 | 744 | 780/60 | 5 |
| PE/Cyanine7 | 565 | 785 | 780/60 | 4 |
| PE/Fire™ 810 | 565 | 806 | Varies. Require cytometers capable of detecting emission signal 780 nm+ off of the red laser. | 4 |
| APC/Cyanine7 | 650 | 785 | 780/60 | 2 |
| APC/Fire™ 750 | 650 | 787 | 780/60 | 2 |
| APC/Fire™ 810 | 650 | 807 | Varies. Require cytometers capable of detecting emission signal 780 nm+ off of the red laser. | 3 |
| PE | Exmax/Emmax | APC | Exmax/Emmax | PerCP | Exmax/Emmax |
| PE/Cyanine5.5 | 565/695 | APC/Cyanine5.5 | 650/695 | PerCP/eFluor® 710 | 480/675 |
| PE/CF594 | 565/610 | APC/H7 | 650/780 | ||
| PE/Texas Red | 565/615 | APC/eFluor® 780 | 650/780 | ||
| PE/Alexa Fluor® 610 | 565/629 | APC/Alexa Fluor ®750 | 650/776 | ||
| PE/Alexa Fluor® 750 | 565/776 | ||||
| View the full listing of trademarks here. | |||||
Other Useful References
- For more information on Brilliant Violet™ polymers, visit Sirigen.
- For more information on tandem families, visit our webpages for Brilliant Violet™, Fire Dyes, and PE/Dazzle™ 594 antibody conjugates.
- First publication on a Brilliant Violet™ tandem, BV570™ :
- Chattopadhyay, P, et al. 2012. Cytometry A. 81:456. Pubmed.
- APC-tandem degradation through a cell dependent mechanism:
- Le Roy, C, et al. 2009. Cytometry A. 75:882. Pubmed.
- Stability of PE-tandem conjugates:
- Hulspas, R, et al. 2009. Cytometry A. 75:966. Pubmed.
- PE/Cyanine5.5 conjugates bind to mouse cells expressing DEC205/CD205:
- Park, CG, et al. 2012. J. Immunol. Methods. 10.1016/j.jim.2012.07.011. Pubmed.
- A technique to block non-specific binding of Cy5 conjugates to monocytes:
- Jahrsdörfer, B, et al. 2005. J. Immunol. Methods. 297:259. Pubmed.
- Binding of PE/Cyanine5 conjugates to the human high-affinity receptor for IgG (CD64):
- Van Vugt, MJ, et al. 1996. Blood. 88:2358. Pubmed.
 Login/Register
Login/Register 







Follow Us