- Regulatory Status
- RUO
- Other Names
- Prostatic acid phosphatase, PAP, 5'-nucleotidase, 5'-NT, Ecto-5'-nucleotidase, Thiamine monophsophatase, TMPase, ACPP
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
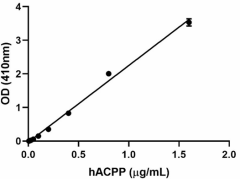
The activity of ACPP is determined by it ability to hydrolyze p-nitrophenyl phosphate (PNPP). The specific activity is > 100,000 pmol/min/µg. -

Stability Testing for Recombinant Human ACPP. Human ACPP was aliquoted in 20 mM Tris, 150 mM NaCl, pH 7.5. One aliquot was frozen and thawed four times (4x Freeze/Thaw) and compared to the control that was kept at 4°C (control). The samples were tested for their ability to hydrolyze p-nitrophenyl phosphate (PNPP). The specific activity is > 100,000 pmol/min/µg.
ACPP, also known as prostatic acid phosphatase, belongs to the histidine acid phosphatase family. It has phosphotyrosyl phosphatase activity. It is predominantly expressed in the prostate gland, and restricted to gladular and ductal epithelial cells, and mainly in the secreted form. It is also widely expressed in non-prostatic tissues, such as bladder, kidney, pancreas, lung, cervix, testis, and ovary, as a type I transmembrane protein with N-terminal phosphatase activity. A mutation at Trp206 reduces ACPP activity, causing its structural instability and increased binding to the L(+)-tartrate inhibitor. The expression of ACPP correlates with prostate cell proliferation. High levels of ACPP leads to slow cell growth. Though the level of ACPP in the circulation is increased in prostate cancer patients, its level and activity are reduced in prostate cancer cells. ACPP serves as a tumor suppressor. Reduced ACPP levels result in hyperphosphorylation of HER-2 (human epidermal growth factor receptor-2), activating downstream MAPK signaling, prostate cancer progression and androgen-independent growth. In addition, ACPP dephosphorylates ErbB-2, blocking downstream ERK1/2 and AKT signaling and tumorigenicity. Several studies indicate that ACPP is cleaved into amyloidogenic fragments, which greatly enhance XMRV (xenotropic murine leukemia virus-releated virus) infection in prostate cancer tissues and HIV infection.
Product DetailsProduct Details
- Source
- Human ACPP, amino acid Lys33-Gln379 (Accession # P15309.3), with a-C-terminal 6His tag, was expressed in 293E cells.
- Molecular Mass
- The 353 amino acid recombinant protein has a predicted molecular mass of approximately 41.12 kD. The DTT-reduced and non-reduced protein migrates at approximately 50 kD by SDS-PAGE. The predicted N-terminal amino acid is Lys.
- Purity
- > 95%, as determined by Coomassie stained SDS-PAGE
- Formulation
- 0.22 µm filtered protein solution is in 20 mM Tris, 150 mM NaCl, pH 7.5.
- Endotoxin Level
- Less than 0.1 EU per µg cytokine as determined by the LAL method
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C for six months. For maximum results, quick spin vial prior to opening. Avoid repeated freeze/thaw cycles.
- Activity
- The activity of ACPP is determined by it ability to hydrolyze p-nitrophenyl phosphate (PNPP). The specific activity is > 100,000 pmol/min/µg.
- Application
-
Bioassay
- Application Notes
-
Human ACPP Activity Assay
- Dilute the recombinant human ACPP at 0.1 µg/mL in assay buffer.
- Dilute the substrate at 2 mM in assay buffer.
- In a plate, combine 50 µL of hACPP and 50 µL of 2 mM Substrate. Include a substrate blank containing 50 µL of assay buffer and 50 µL of 2 mM substrate.
- Incubate the reaction at room temperature for 5 minutes.
- Add 100 µL of 0.2 M NaOH to stop the reaction and develop the color.
- Read the absorbance in endpoint mode at 410 nm.
- Calculate the specific activity:
Specific Activity (pmol/min/µg)= ((Adjusted Vmax* (OD/min) x Conversion Factor** (pmol/OD)) Incubation time (min) x amount of enzyme (µg)
*Adjusted for substrate blank
**Derived using calibration standard p-Nitrophenol (Sigma-Aldrich, Catalog No. 241326).
Per Well:
• Recombinant human ACPP: 0.005 µg
• Substrate: 0.5 mM
Materials
• Assay buffer: 50 mM NaOAc, pH 4.5
• Recombinant human Prostatic Acid Phosphatase/ACPP (hACPP)
• Substrate: p-Nitrophenyl phosphate (Sigma-Aldrich, Catalog No. N2765)
• 96-well clear plate (Costar, Catalog No. 92592)
• Plate reader (Model: SpectraMax Plus by Molecular Devices) or equivalent)
• NaOH, 0.2 M in deionized water
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Monomer
- Distribution
-
Secreted by epithelial cells and synthesized under androgen regulation. Highly expressed in the prostate, also detectable in the bladder, kidney, pancreas, lung, ovary, testis, lung, and cervix; secreted, lysosome membrane, and plasma membrane
- Function
- ACPP is a non-specific phosphatase. It has phosphotyrosyl phosphatase activity.
- Interaction
- Dephosphorylates ERBB2
- Ligand/Receptor
- Converts orthophosphoric monoester to alcohol and orthophosphate
- Bioactivity
- ACPP hydrolyzes p-nitrophenyl phosphate (PNPP).
- Cell Type
- Epithelial cells
- Biology Area
- Cancer Biomarkers, Cell Biology, Immuno-Oncology
- Molecular Family
- Enzymes and Regulators, Proteases
- Antigen References
-
- Lin MF, Clinton GM. 1986. Biochem. J. 235:351.
- Schröder B et al. 2007. Traffic 8:1676.
- Quintero IB, et al. 2007. Cancer Res. 67:6549.
- Veeramani S, et al. Endocr. Relat. Cancer 12:805.
- Zhang Z et al. 1997. Acta Biochim Pol. 44: 659.
- Hong S, et al. 2009. J. Virol. 83:6995.
- Münch J, et al. 2007. Cell 131:1059.
- Chuang TD, et al. 2010. J. Biol. Chem. 285:23598.
- Gene ID
- 55 View all products for this Gene ID
- UniProt
- View information about ACPP on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 








Follow Us