- Regulatory Status
- RUO
- Other Names
- Fibroblast Activation Protein Alpha, FAPalpha, Serine Integral Membrane Protease, DPPIV, Separase, Prolyl Endopeptidase FAP, Dipeptidyl Peptidase FAP, Surface-Expressed Protease, Post-Proline Cleaving Enzyme Fibroblast activation protein
- Ave. Rating
- Submit a Review
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 787302 | 10 µg | 128€ | ||||
| 787304 | 25 µg | 268€ | ||||
Fibroblast activation protein α (FAP) is a type II transmembrane serine protease that can be shed into plasma. FAP is a proline-specific enzyme and is part of the S9 family that includes other proline-specific proteases such as dipeptidyl peptidase IV (DPPIV), DPP8, DPP9, and prolyl endopeptidase (PREP). FAP and dipeptidyl peptidase 4 (DPP4) share similar structural folds, featuring an α/β-hydrolase domain and an eight bladed β-propeller domain. Known DPP4 dipeptides are cleaved by FAP with a 100-fold decrease in catalytic efficiency compared with DPP4. In addition to dipeptidyl peptidase activity, it possesses endopeptidase activity capable of cleaving gelatin. Collagen type I is resistant to the action of FAP, unless it is denatured. It has been implicated in tumor growth and metastasis, and in extracellular matrix remodeling. FAP is expressed in the majority of epithelial cancer-associated fibroblasts, in bone and soft tissue sarcomas, in granulation tissue of healing wounds, rheumatoid arthritis, osteoarthritis, liver disease, inflammatory bowel diseases, and idiopathic pulmonary fibrosis. The shedded FAP in plasma can cleave α-2-antiplasmin (Serpin F2) into a more active form. FGF21 is also a substrate for FAP, and the cleavage produces inactivation of FGF21.
Product DetailsProduct Details
- Source
- Mouse FAP, amino acid Leu26-Asp761 (Accession # NM_007986.2) was expressed in CHO cells. The carboxy terminus contains an -8His tag.
- Molecular Mass
- The 749 amino acid recombinant protein has a predicted molecular mass of approximately 86.7 kD. The DTT-reduced and non-reduced protein migrates at approximately 100 kD by SDS-PAGE. The predicted N-terminal amino acid is Leu.
- Purity
- >95%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in 16 mM Tris, 240 mM NaCl, 20% Glycerol, pH 7.5
- Endotoxin Level
- Less than 1.0 EU per µg protein as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C for six months. For maximum results, quick spin vial prior to opening. Avoid repeated freeze/thaw cycles.
- Activity
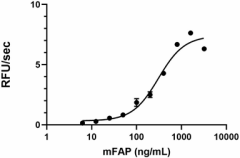
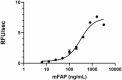
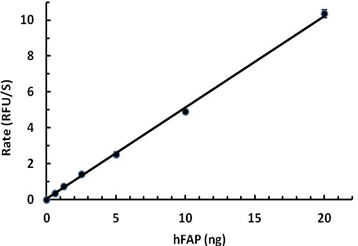
- Mouse FAP, at 0.010 µg/100 µL reaction, cleaves a fluorogenic substrate Z-Gly-Pro-AMC with a specific activity value > 2000 pmol/µg/min.
- Application
-
Bioassay
- Application Notes
-
Mouse FAP Activity assay
Mouse FAP activity is measured by its ability to cleave a fluorogenic peptide substrate Z-Gly-Pro-AMC. The increase of the product formation is monitored by an increase in intensity of fluorescence at 460 nm with excitation at 380 nm.
Materials- Assay Buffer: pH 7.5, 50 mM Tris, 1.0 M NaCl, 0.1% BSA (w/v)
- Recombinant Mouse FAP
- FAP substrate: Z-Gly-Pro-AMC
Activity assay procedures
- Dilute mFAP in the assay buffer at 0.2 µg/mL.
- Dilute the substrate at 100 µM in Assay Buffer.
- Load into a well plate 50 µL of the 0.2 ng/µL mFAP and start the reaction by adding 50 µL of 100 µM Substrate. Include a substrate blank containing 50 µL of Assay Buffer and 50 µL of 100 µM Substrate solution.
- Read the product formation by measuring 380/460 nm (Excitation/Emission) in kinetic mode for 5 minutes.
- The final mFAP concentration is 0.01 µg/100 µL reaction.
- The substrate is 50 µM.
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Monomer
- Distribution
-
Activated fibroblasts associated with tissue remodeling, high levels in mesenchymal cells during embryogenesis. Expressed in bone marrow mesenchymal stem cells.
- Function
- Extracellular matrix degradation, tissue remodeling, fibrosis, wound healing, inflammation, and tumor growth and metastasis. Regulates collagen turnover.
- Ligand/Receptor
- FGF21, Serpin F2 (alpha-2-antiplasmin), Gelatin
- Bioactivity
- Mouse FAP cleaves a fluorogenic substrate Z-Gly-Pro-AMC, FGF21, Serpin 2, and gelatin.
- Cell Type
- Fibroblasts, Mesenchymal cells, Mesenchymal Stem Cells
- Biology Area
- Cancer Biomarkers, Immuno-Oncology, Immunology
- Molecular Family
- Enzymes and Regulators, Proteases
- Antigen References
-
- Scanlan MJ, et al. 1994. Proc Natl Acad Sci U S A. 91:5657-61.
- Piñeiro-Sánchez ML, et al. 1997. J Biol Chem. 272:7595.
- Edosada CY, et al. 2006. FEBS Lett. 580:1581.
- Lee KN, et al. 2006. Blood. 107:1397.
- Bae S, et al. 2008. Br J Haematol. 142:827.
- Fan MH, et al. 2016. J Biol Chem. 291:8070.
- Chen L, et al. 2017. Biochem Biophys Res Commun. 487:8.
- Egger C, et al. 2017. Eur J Pharmacol. 809:64.
- Bainbridge TW, et al. 2017. Sci Rep. 7:12524.
- Gene ID
- 14089 View all products for this Gene ID
- UniProt
- View information about FAP on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
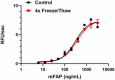
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)


 Login / Register
Login / Register 












Follow Us