- Clone
- XMG1.2 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Interferon-γ, Immune interferon, Type II interferon, T cell interferon, Macrophage-activating factor (MAF)
- Isotype
- Rat IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
IFN-γ is a potent multifunctional cytokine which is secreted primarily by activated NK cells and T cells. Originally characterized based on anti-viral activities, IFN-γ also exerts anti-proliferative, immunoregulatory, and proinflammatory activities. IFN-γ can upregulate MHC class I and II antigen expression by antigen-presenting cells.
Product DetailsProduct Details
- Verified Reactivity
- Mouse
- Antibody Type
- Monoclonal
- Host Species
- Rat
- Immunogen
- E. coli-expressed, recombinant mouse IFN-γ
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- Less than 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
ELISA Capture, ELISPOT Capture - Quality tested
CyTOF® - Verified
Neut, ICFC, IHC, WB - Reported in the literature, not verified in house - Recommended Usage
-
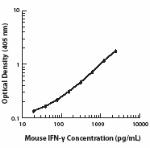
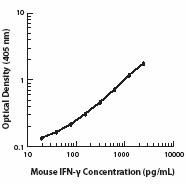
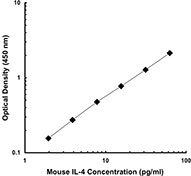
Each lot of this antibody is quality control tested by ELISA assay. For ELISPOT Capture, the suggested use of this antibody is 1 - 4 µg/ml. For ELISA capture applications, a concentration range of 0.5-2.0 µg/ml is recommended. To obtain a linear standard curve, serial dilutions of IFN-γ recombinant protein ranging from 2000 to 15 pg/ml are recommended for each ELISA plate. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
ELISA1-4,11,14 or ELISPOT5 Detection: The biotinylated XMG1.2 antibody is useful as a detection antibody for a sandwich ELISA or ELISPOT assay, when used in conjunction with purified R4-6A2 antibody (Cat. No. 505702/505706) as the capture antibody and recombinant mouse IFN-γ (Cat. No. 575309) as the standard.
ELISA or ELISPOT Capture: The purified XMG1.2 antibody is useful as a capture antibody for a sandwich ELISA or ELISPOT assay, when used in conjunction with biotinylated R4-6A2 antibody (Cat. No. 505704) as the detection antibody and recombinant mouse IFN-γ (Cat. No. 575309) as the standard. The LEAF™ purified antibody is suggested for ELISPOT capture (Cat. No. 505812).
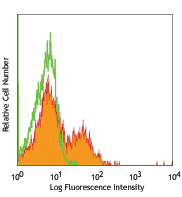
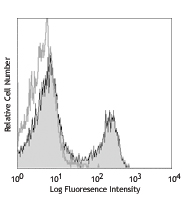
Flow Cytometry7,8,12,13,16: The fluorochrome-labeled XMG1.2 antibody is useful for intracellular immunofluorescent staining and flow cytometric analysis to identify IFN-γ-producing cells within mixed cell populations.
Neutralization1-3,9,10: The XMG1.2 antibody can neutralize the bioactivity of natural or recombinant IFN-γ. The LEAF™ purified antibody (Endotoxin <0.1 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for neutralization of mouse IFN-γ bioactivity in vivo and in vitro (Cat. No. 505812). For in vivo studies or highly sensitive assays, we recommend Ultra-LEAF™ purified antibody (Cat. No. 505834) with a lower endotoxin limit than standard LEAF™ purified antibodies (Endotoxin <0.01 EU/µg).
Additional reported applications (for the relevant formats) include: Western blotting, immunohistochemical staining of frozen tissue sections6,22,23, and immunocytochemistry.
Note: For testing mouse IFN-γ in serum, plasma or supernatant, BioLegend's ELISA Max™ Sets (Cat. No. 430801 to 430806) are specially developed and recommended. - Additional Product Notes
-
Get a 50% discount on this product when purchased in our Activation Bundles. Restrictions apply. Learn more…
-
Application References
(PubMed link indicates BioLegend citation) -
- Abrams J, et al. 1992. Immunol. Rev. 127:5. (ELISA, Neut)
- Sander B, et al. 1993. J. Immunol. Meth. 166:201. (ELISA, Neut)
- Abrams J, et al. 1995. Curr. Prot. Immunol. John Wiley and Sons, New York. Unit 6.20. (ELISA, Neut)
- Yang X, et al. 1993. J. Immunoassay 14:129. (ELISA)
- Klinman D, et al. 1994. Curr. Prot. Immunol. John Wiley and Sons, New York. Unit 6.19. (ELISPOT)
- Sander B, et al. 1991. Immunol. Rev. 119:65. (IHC)
- Ferrick D, et al. 1995. Nature 373:255. (FC)
- Ko SY, et al. 2005. J. Immunol. 175:3309. (FC) PubMed
- Peterson KE, et al. 2000. J. Virol. 74:5363. (Neut)
- DeKrey GK, et al. 1998. Infect. Immun. 66:827. (Neut)
- Dzhagalov I, et al. 2007. J. Immunol. 178:2113. (ELISA)
- Lawson BR, et al. 2007. J. Immunol. 178:5366. (FC)
- Lee JW, et al. 2006. Nature Immunol. 8:181. (FC) PubMed
- Xu G, et al. 2007. J. Immunol. 179:5358. (ELISA) PubMed
- Montfort M, et al.2004. J. Immunol. 173:4084. PubMed
- Haring JS, et al. 2008. J. Immunol. 180:2855. (FC) PubMed
- Jordan JM, et al. 2008. Infect Immun. 76:3717. PubMed
- Tonkin DR, et al. 2008. J. Immunol. 181:4516. PubMed
- Charles N, et al. 2010. Nat. Med. 16:701. (FC) PubMed
- Cui Y, et al. 2009. Invest. Ophth. Vis. Sci. 50:5811. (FC) PubMed
- Mykkanen OT, et al. 2014. PLoS One. 9:114790. PubMed
- Yokogawa M, et al. 2013. Mol. Carcinog. 52:760. (IHC)
- Mottram PL, et al. 1998. J Immunol. 161:602. (IHC)
- Product Citations
-
- RRID
-
AB_11147371 (BioLegend Cat. No. 505833)
AB_11150776 (BioLegend Cat. No. 505834)
AB_2616675 (BioLegend Cat. No. 505847)
AB_2616676 (BioLegend Cat. No. 505848)
AB_2749890 (BioLegend Cat. No. 505857)
AB_2749891 (BioLegend Cat. No. 505858)
Antigen Details
- Structure
- Cytokine; dimer; 40-80 kD (Mammalian)
- Bioactivity
- Antiviral/antiparasitic activities; inhibits proliferation; enhances MHC class I and II expression on APCs
- Cell Sources
- CD8+ and CD4+ T cells, NK cells
- Cell Targets
- T cells, B cells, macrophages, NK cells, endothelial cells, fibroblasts
- Receptors
- IFN-γRα (CDw119) dimerized with IFN-γRβ (AF-1)
- Cell Type
- Tregs
- Biology Area
- Cell Biology, Immunology, Neuroinflammation, Neuroscience
- Molecular Family
- Cytokines/Chemokines
- Antigen References
-
1. Fitzgerald K, et al. Eds. 2001. The Cytokine FactsBook. Academic Press, San Diego.
2. De Maeyer E, et al. 1992. Curr. Opin. Immunol. 4:321.
3. Farrar M, et al. 1993. Annu. Rev. Immunol. 11:571.
4. Gray P, et al. 1987. Lymphokines 13:151. - Regulation
- Upregulated by IL-2, FGF-basic, EGF; downregulated by 1-α-25-Dihydroxy vitamin D3, dexamethasone
- Gene ID
- 15978 View all products for this Gene ID
- Specificity (DOES NOT SHOW ON TDS):
- IFN-gamma
- Specificity Alt (DOES NOT SHOW ON TDS):
- IFN-γ
- App Abbreviation (DOES NOT SHOW ON TDS):
- ELISA Capture,ELISPOT Capture,CyTOF®,Neut,ICFC,IHC,WB
- Hidden Names (DOES NOT SHOW ON TDS):
- IFN-g, IFN-gamma, IFNgamma, IFN gamma, IFNgamma, IFN-gamma, IFNg, IFN-g
- UniProt
- View information about IFN-gamma on UniProt.org
Related FAQs
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Other Formats
View All IFN-γ Reagents Request Custom ConjugationCustomers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
APC anti-mouse IFN-γ
PMA/Ionomycin-stimulated (6hrs) C57BL/6 mouse splenocytes st... -
Biotin anti-mouse IFN-γ
-
FITC anti-mouse IFN-γ
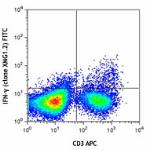
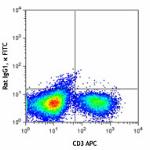
-
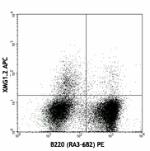
PE anti-mouse IFN-γ

PMA/Ionomycin-stimulated BALB/c T-cells were stained with CD... -
Purified anti-mouse IFN-γ

-
Alexa Fluor® 488 anti-mouse IFN-γ
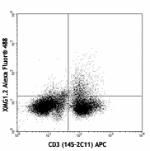
PMA+Ionomycin-stimulated (6hrs) Balb/c mouse splenocytes sur... -
Alexa Fluor® 647 anti-mouse IFN-γ
-
Pacific Blue™ anti-mouse IFN-γ
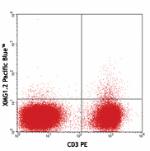
PMA and Ionomycin-stimulated (6hrs) BALB/c splenocytes stain... -
PerCP/Cyanine5.5 anti-mouse IFN-γ

PMA/Ionomycin-stimlated ( 6hrs ) C57BL/6 splenocytes surface... -
PE/Cyanine7 anti-mouse IFN-γ
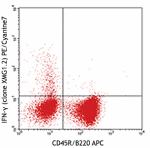
PMA/Ionomycin-stimulated (6hrs) C57BL/6 mouse splenocytes st... -
Brilliant Violet 421™ anti-mouse IFN-γ
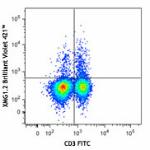
C57BL/6 mouse splenocytes were stimulated with PMA + Ionomyc... 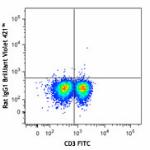
-
Brilliant Violet 650™ anti-mouse IFN-γ
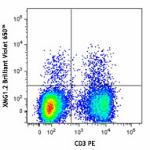
PMA + Ionomycin-stimulated (6 hours) C57BL/6 mouse splenocyt... -
Ultra-LEAF™ Purified anti-mouse IFN-γ

-
Brilliant Violet 711™ anti-mouse IFN-γ
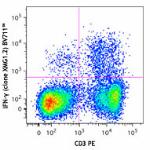
PMA + Ionomycin-stimulated (6 hours) C57BL/6 mouse splenocyt... -
Brilliant Violet 785™ anti-mouse IFN-γ
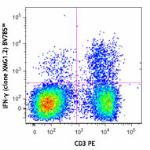
PMA + Ionomycin-stimulated (6 hours) C57BL/6 mouse splenocyt... -
Brilliant Violet 605™ anti-mouse IFN-γ
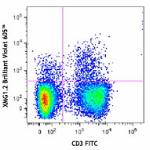
PMA+ionomycin-stimulated C57BL/6 mouse splenocytes (6 hours,... -
Brilliant Violet 510™ anti-mouse IFN-γ
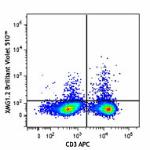
PMA+ionomycin-stimulated C57BL/6 mouse splenocytes (6 hours,... -
Purified anti-mouse IFN-γ (Maxpar® Ready)
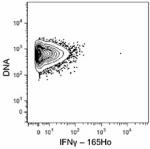
C57BL/6 mouse splenocytes were incubated for 6 hours in medi... 
-
PE/Dazzle™ 594 anti-mouse IFN-γ
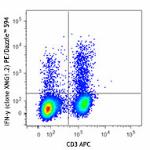
PMA + Ionomycin-stimulated (6 hours) C57BL/6 mouse splenocyt... 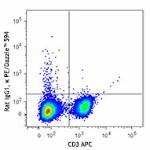
-
Alexa Fluor® 700 anti-mouse IFN-γ
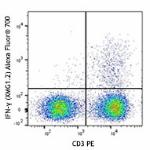
C57BL/6 mouse splenocytes were stimulated with Cell Activati... 
-
APC/Cyanine7 anti-mouse IFN-γ

C57BL/6 mouse splenocytes were stimulated with Cell Activati... -
GoInVivo™ Purified anti-mouse IFN-γ
-
APC/Fire™ 750 anti-mouse IFN-γ

C57BL/6 mouse splenocytes stimulated with PMA+ Ionomycin in ... -
Spark NIR™ 685 anti-mouse IFN-γ

C57BL/6 mouse splenocytes stimulated with PMA + Ionomycin in... -
Spark UV™ 387 anti-mouse IFN-γ

C57BL/6 mouse splenocytes stimulated with PMA+ Ionomycin in ... -
Brilliant Violet 750™ anti-mouse IFN-γ

PMA+ ionomycin stimulated C57BL/6 mouse splenocytes (6 hours...











Follow Us