- Clone
- TAU-13 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Microtubule-associated protein tau, PHF-tau, paired helical filament-tau, neurofibrillary tangle protein, microtubule-associated protein tau, isoform 4, G protein beta1/gamma2 subunit-interacting factor 1
- Previously
-
Covance Catalog# MMS-520R
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
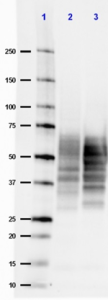
Western blot of anti-Tau, 15-25 antibody (clone TAU-13). Lane 1: Molecular weight marker; Lane 2: 20 µg of human Alzheimer's disease brain lysate; Lane 3: 20 µg of normal human brain lysate. The blot was incubated with a 1:500 dilution of the primary antibody overnight at 4°C, followed by incubation with HRP labeled goat anti-mouse IgG (Cat. No. 405306). Enhanced chemiluminescence was used as the detection system.
Tau proteins are microtubule-associated protein (MAPs) which are abundant in neurons of the central nervous system, but are also expressed at very low levels in CNS astrocytes and oligodendrocytes and elsewhere. One of tau's main functions is to modulate the stability of axonal microtubules. Tau is active primarily in the distal portions of axons providing microtubule stabilization as well as flexibility. Pathologies and dementias of the nervous system such as Alzheimer's disease feature tau proteins that have become defective and no longer stabilize microtubules properly. As a result, tau forms aggregates with specific structural properties referred to as Paired Helical Filaments (PHFs) that are a characteristic of many different types of dementias, known as tauopathies. Tau has two primary ways of controlling microtubule stability: isoforms and phosphorylation. Six tau isoforms exist in human brain tissue, and they are distinguished by the number of binding domains. Three isoforms have three binding domains and the remaining three have four binding domains. The binding domains are located in the carboxy-terminus of the protein and are positively-charged (for binding to the negatively-charged microtubule). Tau isoforms with four binding domains are better at stabilizing microtubules than those with three binding domains. Thus, in the human brain, the tau proteins constitute a family of six isoforms with the range from 352-441 amino acids. They also differ in either zero, one or two inserts of 29 amino acids at the N-terminal part (exon 2 and 3), and three or four repeat-binding regions at the C-terminus. So, the longest isoform in the CNS has four repeats (R1, R2, R3 and R4) and two inserts (441 amino acids total), while the shortest isoform has three repeats (R1, R3 and R4) and no insert (352 amino acids total). Tau is also a phosphoprotein with 79 potential Serine (Ser) and Threonine (Thr) phosphorylation sites on the longest tau isoform. Phosphorylation has been reported on approximately 30 of these sites in normal tau proteins. Mechanisms that drive tau lesion formation in the highly prevalent sporadic form of AD are not fully understood, but appear to involve abnormal post-translational modifications (PTMs) that influence tau function, stability, and aggregation propensity.
Product DetailsProduct Details
- Verified Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Preparation
- Ascites
- Concentration
- The concentration is not quantified as this product is sold as undiluted crude mouse ascites fluid. The concentration might vary from lot-to-lot and an estimated concentration would be 1-3 mg/ml.
- Storage & Handling
- Store at -20°C or below. Upon initial thawing, apportion into working aliquots and store at -20°C or below. Avoid repeated freeze-thaw cycles to prevent denaturing the antibody. Do not store in frost-free freezers.
- Application
-
WB - Quality tested
IHC-Other - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by western blotting. For western blotting, a dilution range of 1:500-1:1000 is suggested. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
TAU-13 is a mouse monoclonal antibody that binds to human tau and is able to stain brain tissue early in Alzheimer’s disease. TAU-13 is specific for human tau; it does not react with bovine, murine or rat tau. Preliminary assignment of its epitope indicates that it maps to amino acid residues 15-25 on the longest isoform of human tau.
This antibody clone has been reported for use on IHC of 4% PFA-fixed free floating sections1. -
Application References
(PubMed link indicates BioLegend citation) -
- Bi M, et al. 2011. PLoS ONE. 6:12. (IHC-other)
- García-Sierra F, et al. 2003. J Alzheimers Dis. 5:65–77. (IHC-other)
- Product Citations
-
- RRID
-
AB_2565341 (BioLegend Cat. No. 835201)
Antigen Details
- Structure
- Unmodified Tau isoforms have an apparent molecular weight ranging from 33-79 kD. Additional high and low molecular weight Tau species have been observed in brain tissues.
- Distribution
-
Tissue distribution: Central nervous system, peripheral ganglia and nerves, kidney, skeletal, and heart muscle.
Cellular distribution: Cytoskeleton, nucleus, plasma membrane, and cytosol. - Function
- Tau promotes microtubule assembly and stability. The short tau isoforms allow plasticity of the cytoskeleton whereas the longer isoforms may preferentially play a role in its stabilization.
- Interaction
- Tau interacts with Sequestosome-1, Peptidyl-prolyl cis-trans isomerase FKBP4, casein kinase I isoform delta, serine/threonine-protein kinase Sgk1, Laforin, and alpha-synuclein.
- Biology Area
- Cell Biology, Neurodegeneration, Neuroscience, Protein Misfolding and Aggregation
- Molecular Family
- Tau
- Antigen References
-
- Castillo-Carranza DL, et al. 2014. J Neurosci. 12:4260. (WB)
- Gene ID
- 4137 View all products for this Gene ID
- UniProt
- View information about Tau 15-25 on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
Other Formats
View All Tau, 15-25 Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Anti-Tau, 15-25 | TAU-13 | WB,IHC |
| Purified anti-Tau, 15-25 | TAU-13 | WB |
Customers Also Purchased
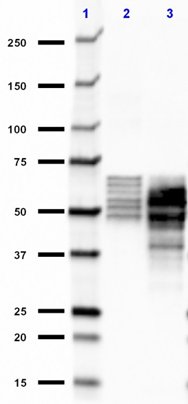

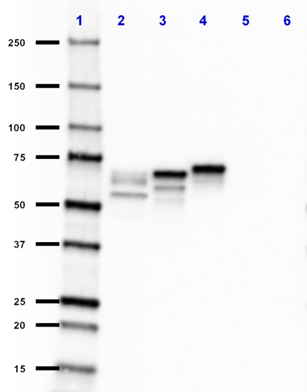
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Anti-Tau, 15-25
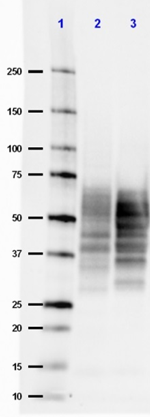
Western blot of anti-Tau, 15-25 antibody (clone TAU-13). Lan... -
Purified anti-Tau, 15-25
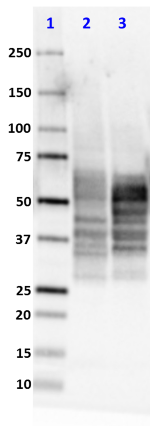
Western blot of purified anti-Tau, 15-25 antibody (clone TAU...












Follow Us