- Clone
- SMI 51 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Microtubule-associated protein tau, PHF-tau, paired helical filament-tau, neurofibrillary tangle protein, microtubule-associated protein tau, isoform 4, G protein beta1/gamma2 subunit-interacting factor 1
- Previously
-
Covance Catalog# SMI-51R
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
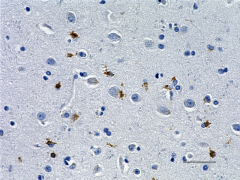
IHC staining of anti-Tau, 95-108 (PHF) antibody (clone SMI 51) on formalin-fixed paraffin-embedded human Alzheimer's disease brain tissue. Following antigen retrieval using Sodium Citrate H.I.E.R., the tissue was incubated with a 1:1000 dilution of the primary antibody for 60 minutes at room temperature. BioLegend's Ultra-Streptavidin (USA) HRP kit (Multi-Species, DAB, Cat. No. 929901) was used for detection followed by hematoxylin counterstaining, according to the protocol provided. The image was captured with a 40X objective. Scale bar: 50 µm -
IHC staining of anti-Tau, 95-108 (PHF) antibody (clone SMI 51) on formalin-fixed paraffin-embedded human Alzheimer's disease brain tissue. Following antigen retrieval using Sodium Citrate H.I.E.R., the tissue was incubated with a 1:1000 dilution of the primary antibody for 60 minutes at room temperature. BioLegend's Ultra-Streptavidin (USA) HRP kit (Multi-Species, DAB, Cat. No. 929901) was used for detection followed by hematoxylin counterstaining, according to the protocol provided. The image was captured with a 40X objective. Scale bar: 50 µm -
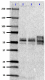
Western blot of anti-Tau, 95-108 (PHF) antibody (clone SMI 51). Lane 1: Molecular weight marker; Lane 2: 20 µg of normal human brain lysate; Lane 3: 20 µg of human Alzheimer´s disease brain lysate; Lane 4: 0.1 µg of recombinant Tau ladder protein. The blot was incubated with a 1:1000 dilution of the primary antibody overnight at 4°C, followed by incubation with HRP labeled goat anti-mouse IgG (Cat. No. 405306). Enhanced chemiluminescence was used as the detection system.
Tau proteins are microtubule-associated protein (MAPs) which are abundant in neurons of the central nervous system, but are also expressed at very low levels in CNS astrocytes and oligodendrocytes and elsewhere. One of tau's main functions is to modulate the stability of axonal microtubules. Tau is active primarily in the distal portions of axons providing microtubule stabilization as well as flexibility. Pathologies and dementias of the nervous system such as Alzheimer's disease feature tau proteins that have become defective and no longer stabilize microtubules properly. As a result, tau forms aggregates with specific structural properties referred to as Paired Helical Filaments (PHFs) that are a characteristic of many different types of dementias, known as tauopathies.
Tau has two primary ways of controlling microtubule stability: isoforms and phosphorylation. Six tau isoforms exist in human brain tissue, and they are distinguished by the number of binding domains. Three isoforms have three binding domains and the remaining three have four binding domains. The binding domains are located in the carboxy-terminus of the protein and are positively-charged (for binding to the negatively-charged microtubule). Tau isoforms with four binding domains are better at stabilizing microtubules than those with three binding domains.
Thus, in the human brain, the tau proteins constitute a family of six isoforms with the range from 352-441 amino acids. They also differ in either zero, one or two inserts of 29 amino acids at the N-terminal part (exon 2 and 3), and three or four repeat-binding regions at the C-terminus. So, the longest isoform in the CNS has four repeats (R1, R2, R3 and R4) and two inserts (441 amino acids total), while the shortest isoform has three repeats (R1, R3 and R4) and no insert (352 amino acids total). Tau is also a phosphoprotein with 79 potential Serine (Ser) and Threonine (Thr) phosphorylation sites on the longest tau isoform. Phosphorylation has been reported on approximately 30 of these sites in normal tau proteins. Mechanisms that drive tau lesion formation in the highly prevalent sporadic form of AD are not fully understood, but appear to involve abnormal post-translational modifications (PTMs) that influence tau function, stability, and aggregation propensity.
Product Details
- Verified Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- Ascites Fluid (contains 0.01M sodium azide).
- Preparation
- Ascites
- Concentration
- The concentration is not quantified as this product is sold as undiluted crude mouse ascites fluid. The concentration might vary from lot-to-lot and an estimated concentration would be 1-3 mg/ml.
- Storage & Handling
- Store at -20°C. Upon initial thawing, apportion into working aliquots and store at -20°C. Avoid repeated freeze-thaw cycles to prevent denaturing the antibody. For long-term storage, keep the antibody at -80°C.
- Application
-
IHC-P - Quality tested
WB - Verified - Recommended Usage
-
Each lot of this antibody is quality control tested by formalin-fixed paraffin-embedded immunohistochemical staining. For immunohistochemistry, the suggested dilution is 1:500 - 1:1000. For Western blotting, the suggested dilution is 1:500 - 1:1000. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
This antibody is effective in immunoblotting (WB) and immunohistochemistry (IHC-P).
SMI 51 reacts with microtubule-associated protein tau independently of its degree of phosphorylation. By IHC, this antibody reacts strongly with paired helical filaments in Alzheimer disease while its reaction with Tau in normal human tissue is relatively weak. The antibody recognizes the 95 to 108 sequence of Tau (AGIGDTSNLEDQAA) in a conformation partially dependent on serine in position 101. - Additional Product Notes
-
NULL
- RRID
-
AB_2565358 (BioLegend Cat. No. 836101)
Antigen Details
- Biology Area
- Cell Biology, Neurodegeneration, Neuroscience, Protein Misfolding and Aggregation
- Molecular Family
- Tau
- Gene ID
- 4137 View all products for this Gene ID
- UniProt
- View information about Tau 95-108 on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
Other Formats
View All Tau, 95-108 (PHF) Reagents Request Custom Conjugation| Description | Clone | Applications |
|---|---|---|
| Anti-Tau, 95-108 (PHF) | SMI 51 | IHC-P,WB |
| Purified anti-Tau, 95-108 (PHF) | SMI 51 | WB,IHC-P |
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Anti-Tau, 95-108 (PHF)
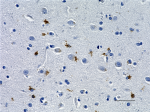
IHC staining of anti-Tau, 95-108 (PHF) antibody (clone SMI 5... 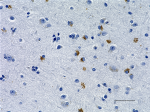
IHC staining of anti-Tau, 95-108 (PHF) antibody (clone SMI 5... 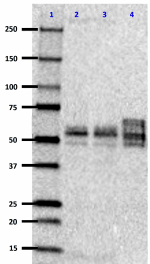
Western blot of anti-Tau, 95-108 (PHF) antibody (clone SMI 5... -
Purified anti-Tau, 95-108 (PHF)
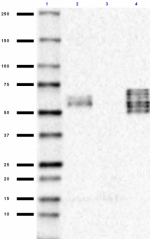
Western blot of purified anti-Tau, 95-108 (PHF) antibody (cl... 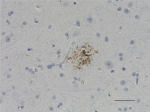
IHC staining of purified anti-Tau, 95-108 (PHF) antibody (cl... 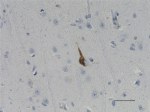
IHC staining of purified anti-Tau, 95-108 (PHF) antibody (cl...













Follow Us